Surgical resection of metastatic pancreatic cancer: is it worth it?—a 15-year experience at a single Chinese center
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive malignancy with a dismal prognosis consisting of a mortality rate approximately equal to its incidence and a 5-year survival rate of around 8% (1). Despite improvements in chemoradiotherapy efficacy, surgical resection remains the sole curative method with the potential to enhance the 5-year survival rate to 20% (2,3). Although surgery for PDAC remains demanding and risky, remarkable progress has now made pancreatic surgery safe to perform with low mortality and morbidity rates (4,5). Some types of surgeries, such as vascular resection and reconstruction, that were previously considered contraindications can now be performed by skilled pancreatic surgeons, who can even attain R0 resection in considerable numbers of patients (5).
Many patients with PDAC present with distal metastasis at the time of diagnosis (6). Moreover, a definite TNM classification can be achieved in some patients only with operative exploration despite the advent of advanced noninvasive detection tools such as computed tomography or positron emission tomography–computed tomography (7,8). Such cases present a treatment dilemma to us, making it unclear whether we should perform metastatic resection of the primary tumor, palliative bypass, or simply close the abdominal area. Unlike hepatectomy for colorectal metastases, which is currently commonly used for colorectal liver metastases with 5-year survival rates of up to 58% (9), according to National Comprehensive Cancer Network (NCCN) guidelines, tumor resection should not be performed when other organs are infiltrated by PDAC (10,11). Thus, metastasectomy of PDAC has long been controversial. In terms of synchronous resection of liver metastases with pancreatectomy, different centers including Heidelberg and Charite-University of Berlin reported different results including perioperative complications and overall survival of 8–39 months (8,12-14). However, early on, these trials considered only liver metastasis and seldom considered other organs. Furthermore, related information was not recorded in detail by some studies, making it difficult to justify the use of metastasectomy for PDAC metastasis.
As one of the largest pancreatic disease centers worldwide, our institution employs several skilled surgeons who perform more than 500 pancreatectomy cases per year. In this context, we collected the clinical data of the long-term survival rate and treatment quality of M1 PDAC patients in past 15 years, important aspects that were rarely reported in previous articles, and evaluated different variants that influenced patient survival and treatment quality.
Methods
Patients
A retrospective review was conducted of all patients with M1 PDAC who were admitted to the Department of Pancreatic Surgery of Ruijin Hospital between January 1, 2003, and December 31, 2014. The patients’ data were entered into a prospective database established in 2003 after their hospital admission. The patients were followed up until the time of death or study completion (December 31, 2017). PDAC was diagnosed according to NCCN criteria, and all the patients had histologically verified PDAC. None of the patients received neoadjuvant chemotherapy or other preoperative anticancer treatment. Metastatic sites were detected in all cases using enhanced computed tomography or magnetic resonance imaging. Gastroscopy and colonoscopy were performed in selected patients.
Some patients were diagnosed preoperatively with stage IV PDAC. Except for patients who refused to receive any treatment, a multidisciplinary clinical assessment was used to evaluate the possibility of resection. For patients who could not be diagnosed preoperatively with stage IV PDAC, exploratory laparotomy was performed with patient and family consent of the patients and their relatives. Patients with non-metastatic PDAC were excluded from this study. The remaining patients were thoroughly evaluated by at least three skilled surgeons to determine the possibility of resection. The patients and their relatives were well informed of the benefits and risks of surgical treatment.
This study was approved by the ethics committee of Ruijin Hospital, which is affiliated with Shanghai Jiao Tong University, in accordance with the latest version of the Declaration of Helsinki. All subjects signed an informed consent form.
Surgical techniques
In a horizontal position, the patients received adequate anesthesia and underwent surgical exploration through a reverse L-shaped abdominal incision. After opening the abdominal cavity, we examined the abdominal wall, omentum majus, mesentery, liver, stomach, small intestine, and intestinal colon to identify possible metastasis and its extent to decide whether it could be synchronously resected. The metastatic sites were validated using intraoperative frozen sections. Intraoperative ultrasonography and preoperative gastrointestinal endoscopy were performed if possible. Synchronous tumor resection was performed if the metastatic site could be removed completely (not wide metastasis). Briefly, a pancreaticoduodenectomy was conducted when the tumor was in the head or neck of the pancreas, while a distal pancreatectomy was employed when the tumor was close to the body or tail of the pancreas (Figure 1).
Synchronous tumor resection was discontinued if multiple metastases were found in the abdominal wall, omentum majus, and mesentery that were not detected preoperatively and were impossible to resect. In terms of the involved intestine and colon, cases of local invasion were considered suitable for resection, while those of widespread metastasis were considered contraindicated. For unresectable cases in the pancreatic head, palliative bypass was performed if jaundice or a gastrointestinal tract obstruction occurred before surgery.
Main outcomes
Collected patient characteristics included age, sex, American Society of Anesthesiologists (ASA) score, body mass index, tumor size, total bilirubin level, albumin level, Child-Pugh classification, CA 19-9 level, tumor size, number of metastatic organs, and number of metastatic vessels. Surgical variables included operative time, intraoperative blood loss, and volume of intraoperatively infused blood. Postoperative variables included morbidity, mortality, and length of postoperative hospital stay. Postoperative morbidities were evaluated according to the Clavien-Dindo classification system, in which a major complication was defined as a score ≥3.
Statistical analyses
All included patients were followed up until death or the end of the clinical trial period on December 31, 2017. The statistical software SPSS 20.0 (SPSS Inc., Chicago, IL, USA) was used in the statistical analyses. Continuous data are expressed as mean ± SD or median. Continuous variables were compared using Student’s t-test and one-way analysis of variance. Categorical variables were compared using the chi-square test or Fisher’s exact probability test. Survival rates were analyzed using the Kaplan-Meier method, and significant differences were determined by using the log-rank test. Patients who died perioperatively were not included in the survival analysis. Prognostic variables were screened through a Cox regression analysis. Multivariate analysis was performed using a Cox proportional hazard model to identify independent prognostic factors.
Results
Patient characteristics
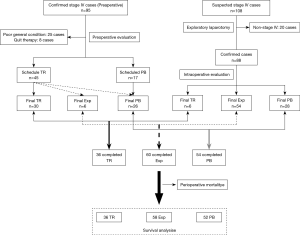
From January 1, 2003 to December 31, 2014, 203 patients with suspicious stage IV pancreatic cancer were diagnosed at our center. Of them, 95 were confirmed preoperatively and 8 patients quit therapy at the time of diagnosis. Multidisciplinary evaluations revealed that 25 patients were unsuitable candidates for aggressive surgical therapy because of a poor general condition. For the remaining 62 patients, synchronous tumor resection was scheduled in 45 and palliative bypass was scheduled in 17. Finally, tumor resection was positive in 30 patients; however, it failed in the other 15, due to an undetectable metastasis requiring palliative bypass (jaundice or gastrointestinal obstruction was noted perioperatively) or the operation was simply terminated (no complication before surgery).
A definite diagnosis could not be made preoperatively in the remaining 108 patients, so exploratory laparotomy was performed. The benefits and risks of synchronous tumor resection were well explained preoperatively to the patients and their relatives, and the possibility of resection was thoroughly evaluated intraoperatively by at least three senior surgeons. Of them, 20 patients were excluded due to having non-stage IV PDAC. Finally, 6 patients underwent synchronous tumor resection, 28 underwent palliative bypass, and 54 underwent surgical termination because of unresectable metastases in the omentum majus, abdominal wall, or mesentery.
Overall, 36 patients underwent synchronous tumor resection, 54 received palliative bypass, and 60 underwent only exploratory laparotomy. In terms of perioperative mortality, none of the patients who underwent synchronous tumor resection died, but 2 patients each who received palliative bypass or exploratory laparotomy died. The patient selection process is shown in Figure 2.
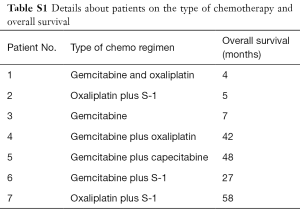
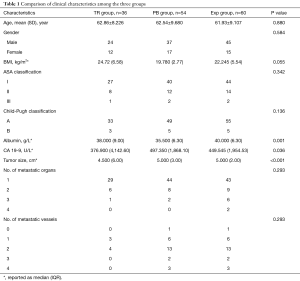
After surgery, 7 of 36 patients received chemotherapy (Table S1). All patients’ baseline characteristics are listed in Table 1. The ASA classification system and Child-Pugh classification were used to evaluate the patients’ general condition. Although significant differences were observed in albumin level, CA 19-9 level, and tumor size, no significant difference was found in Child-Pugh classification or other parameters.
Full table
Full table
Surgical outcomes
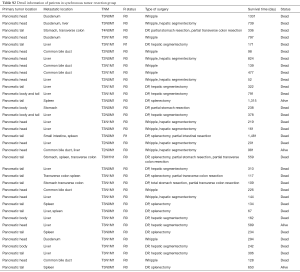
Of the 36 patients who underwent synchronous tumor resection, 17 had pancreatic head cancer and 19 had pancreatic body or tail cancer. Pancreatic tumor and metastatic sites were extirpated completely in all cases, including 7 with two or three metastatic sites. Information on the primary tumor location, metastatic site, TNM stage, resection extent, incision margin, and survival status are presented in Table S2. A total of 17 patients underwent hepatic segmentectomy; 4 underwent gastrectomy (1 total gastrectomy); 4 underwent partial colectomy; and 1 underwent partial small intestinal resection. To prevent tumor recurrence, all patients with pancreatic body and tail cancer also underwent a splenectomy.
Full table
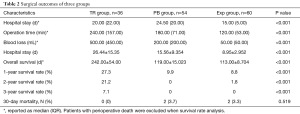
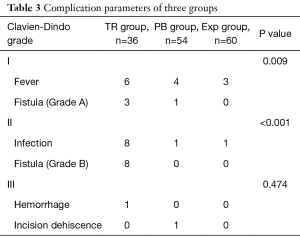
The surgical data of the patients in the three therapeutic model are presented in Table 2. The median operative time was significantly longer and intraoperative blood loss was higher in the synchronous tumor resection group than in the other two groups. No perioperative deaths occurred in the tumor resection patients, whereas 2 deaths occurred in the palliative bypass and exploratory laparotomy groups. Postoperative complications occurred in 26 patients in the tumor resection group. However, only 1 patient developed a major complication (postoperative bleeding), which was treated with digital subtraction angiography; 6 had fever; and 11 developed a pancreatic fistula (grade A in 3, grade B in 8) and received conservative treatment. Although the complication rates were higher in the tumor resection group than in the other two groups, the severity of the complications did not significantly differ. Details of the postoperative complications are listed in Table 3.
Full table
Full table
We divided the metastatic sites into two types. The first type is metastasis to adjacent organs such as the common bile duct, duodenum, and spleen. Although this type of metastasis occurs locally and could be removed by standard surgical resection in most cases, it is associated with worse prognosis than non-metastatic cases (data not shown). The other type is distant metastasis such as those to the liver, stomach, and colon. For surgeons, this is the most difficult type of metastasis to manage. According to our 12-year experience, metastatic sites are the basis for deciding whether resection is possible; thus, they are highly related with prognosis. In our survival analysis, we found that, except for omentum majus involvement, the involvement of other organs was not positively correlated with prognosis. This means that metastasis to the omentum majus is a better predictor of a possible worse prognosis than metastases to other organs (Table 4). This is theoretically easy to understand since the operation was stopped if omentum majus metastasis was found during the exploratory laparotomy; however, some micrometastases were difficult to identify even intraoperatively and could be only validated by postoperative pathological examination. Although the omentum majus was regularly resected in this surgery, the PDAC had already metastasized.
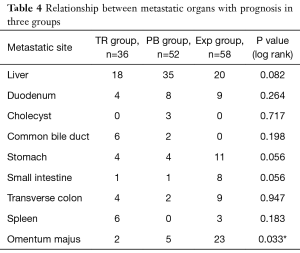
Full table
Survival analysis
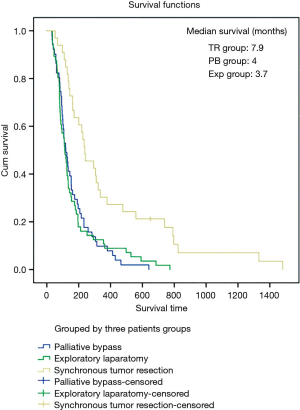
The 1-, 2-, and 3-year survival rates of the synchronous tumor resection group were 27.3%, 21.2%, and 7.1%, respectively, with a median survival time of 7.9 months. The mean survival rates of the patients in the palliative bypass group were 9.8% at 1 year and 0% at 2 and 3 years. Their median survival time was 4 months. The survival rates of the patients in the exploratory laparotomy group were 8.8%, 1.8%, and 0% at 1, 2, and 3 years, respectively. Their median survival time was 3.7 months (Figure 3). The median survival time of palliative bypass group and exploratory laparotomy group were significantly shorter than synchronous tumor resection group (P<0.05).
In the subsequent analysis, we compared the survival time of patients with metastasis to the liver versus other organs since the liver was the most frequently metastatic target reported in previous studies from other institutes. The median survival time was 4.4 months in liver metastasis group versus 4.5 months in the other organ’s metastasis group, showing no significant intergroup difference (P=0.245).
To assess the correlation between overall number of metastasis organs and survival, we divided the patients into four groups. The median survival times were 4.6, 4, 3.8, and 1.2 months in the one, two, three, and four metastasis organ groups, respectively. The survival time of the four metastases organs group was significantly shorter than those of the other groups (P<0.001). Considering the development of disease, the metastasis to four organs will negatively impact prognosis, which may be attributed to tumor burden.
In the univariate analysis of patients who underwent synchronous tumor resection, several parameters were included (Table 5); however, no significant factor was correlated with overall survival. In the multivariate analysis (Table 6), tumor size larger than 2 cm was an independent risk factor (P=0.033; hazard ratio, 2.4; 95% confidence interval, 1.075–5.363). Although age was primarily considered another risk factor, no significant difference was found to be correlated with overall survival.
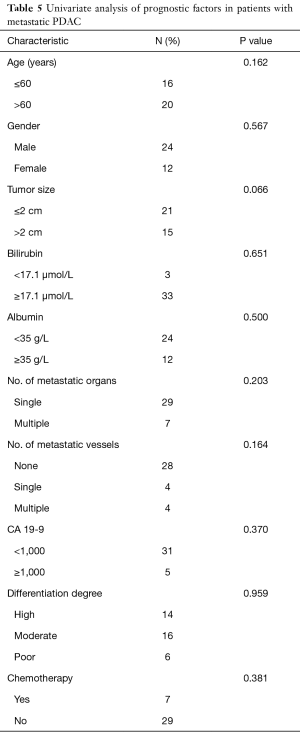
Full table
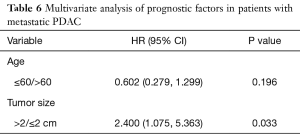
Full table
Discussion
PDAC still leads to dismal prognosis since surgery is the only potentially curative method available for resectable pancreatic cancer. Reports have shown that longer survival time could be achieved after curative resection (R0 resection) and positive node clearance (14,15). Unfortunately, according to the current guidelines, complete resection can be achieved in few patients due to local or distant metastasis (16). The guidelines of PDAC therapy have changed over time from radical resection to extensive lymph node dissection to chemotherapy preference. Based on the present understanding of PDAC, other palliative treatments such as chemotherapy or radiotherapy are recommended to tackle metastatic PDAC. However, palliative chemotherapy, mainly gemcitabine monotherapy, the classic regimen for nearly 20 years, provided only limited benefits with a median survival time of 5.0–7.2 months (17,18). Nab-paclitaxel, a new regimen, reportedly had better prognosis, with a median survival time of 8.5 months when administered synchronously with gemcitabine (19). The combined use of different chemotherapeutics such as the FOLFIRINOX regimen may improve survival time to a median 11.1 months. However, in addition to its higher cost, FOLFIRINOX carries serious side effects, which restricts its clinical application (16), especially in China. Therefore, investigations into the appropriate treatment are urgently required.
Due to the fact that most patients with PDAC have occult or overt metastasis, performing pancreatic resection together with metastasectomy, as in the case of hepatic metastasis for colorectal cancer, may be worthwhile, although it seems contradictory to the current PDAC treatment guidelines. Some large institutions have already attempted to perform hepatic metastasectomy synchronous with pancreatic tumor resection, leading to different arguments (Table S3) (7,12-14,20). Some scholars reported an overall survival time of 13.8 months, with a 1-year survival rate of 58.9% after synchronous hepatectomy for M1 pancreatic cancer patients, highly supporting the proposal that synchronous hepatectomy for these patients would prolong the overall survival time (7). Other scholars, on the other hand, were strongly against extended resection in cases of hepatic metastasis. Their results indicated that not only the overall survival did not improve, but surgery-related morbidity and mortality increased (14). Although these studies recommended against this surgery, they admitted that synchronous hepatic resection of M1 PDAC could be performed safely by skilled surgeons at appropriate institutions.

Full table
Whether neoadjuvant and adjuvant radiotherapy or chemotherapy were used or standardized in these patients in previous studies was unclear. Furthermore, these studies considered only liver metastasis. However, we should realize that although the liver is the most common metastatic organ in PDAC, it is not the only possible metastatic site (Table S2). Thus, in the current study, we included only patients who did not receive neoadjuvant chemotherapy as subjects and considered metastasis to other organs. Furthermore, multidisciplinary clinical assessments were performed of every patient to decrease selection bias and clarify the benefits of surgery. We also analyzed the data of the patients who underwent only palliative bypass or exploratory laparotomy. Our study’s purpose was not to clarify whether synchronous tumor resection was superior to palliative treatment; rather, we aimed to clarify whether tumor burden could vary among patients with stage IV PDAC treated according to NCCN guidance and should they be treated differently. Of our cohort, 36 patients in the synchronous tumor resection group may have had a lower tumor burden than those in the other two groups; thus they could benefit from surgery since they had a relatively longer overall survival time of 7.9 months, not too much worse than those who underwent gemcitabine plus nab-paclitaxel treatment (8.5 months). Patients with more than four metastasis organs showed a shorter survival time than others, which also reminds us of taking a cautious attitude toward treating these patients surgically. We plan to perform a randomized controlled study to evaluate whether their overall survival time would be prolonged by the combination use of synchronous tumor resection and (neo)adjuvant chemotherapy since chemotherapy currently plays a more important role than ever before but also has its limitations.
Our study has some limitations. First, selection bias and some confounding factors exist, and we were unable to use propensity score matching analysis to compare the outcomes of the three different groups due to the small number of samples. Furthermore, it was a retrospective study instead of a randomized controlled trial; thus, its level of evidence is not that high. Second, although we ensured that none of our patients received neoadjuvant radiotherapy or chemotherapy before surgery, we could not control for the proportion of patients who received adjuvant therapy after surgery, even though it did not influence overall survival after the statistical analysis since few patients received chemotherapy due to its treatment heterogeneity and side effects. Finally, the number of patients in this study was not large, which may limit the strength of our results. On the one hand, this is a high-risk and controversial surgery, so we could not persuade patients to undergo it. On the other hand, there is still no clear protocol for selecting appropriate patients to undergo this surgery due to cancer heterogeneity and differences in tumor burden.
Based on the current knowledge, we will start further studies as follows. First, we will investigate new therapies targeting patients with metastasis. Since synchronous tumor resection is feasible in selected patients, we will combine intraoperative radiotherapy and postoperative personalized chemotherapy. Second, we found that synchronous tumor resection could be performed in selected patients. However, identifying appropriate patients is challenging. Thus, a clinical system should be developed to evaluate the possibility of resection in affected patients.
Conclusions
Although technically challenging, surgical resection for metastatic PDAC can be safely performed in and may prolong the overall survival time of some highly selected patients. Omentum majus metastasis is a predictor of worse prognosis in M1 PDAC patients compared to another organ metastasis. Primary tumor size may be a factor for deciding whether appropriate M1 PDAC patients could benefit from synchronous tumor resection. Although the surgery was safely performed in this study, it cannot be widely recommended until high-quality randomized controlled trials are conducted. Furthermore, whether surgery combined with adjuvant or neoadjuvant chemotherapy may further prolong overall survival in this kind of patient cohort remains to be determined.
Acknowledgments
The authors thank Wei Wang and Meng Yang, the other doctor and the secretary of our center, for managing the study data.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo.2020.03.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the ethics committee of Ruijin Hospital, which is affiliated with Shanghai Jiao Tong University, in accordance with the latest version of the Declaration of Helsinki. All the subjects signed an informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Benassai G, Quarto G, Perrotta S, et al. Long-term survival after curative resection for pancreatic ductal adenocarcinoma--Surgical treatment. Int J Surg 2015;21 Suppl 1:S1-3. [Crossref] [PubMed]
- Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg 2003;237:74-85. [Crossref] [PubMed]
- Riediger H, Adam U, Utzolino S, et al. Perioperative outcome after pancreatic head resection: a 10-year series of a specialized surgeon in a university hospital and a community hospital. J Gastrointest Surg 2014;18:1434-40. [Crossref] [PubMed]
- Chua TC, Saxena A. Extended pancreaticoduodenectomy with vascular resection for pancreatic cancer: a systematic review. J Gastrointest Surg 2010;14:1442-52. [Crossref] [PubMed]
- Lennon AM, Wolfgang CL, Canto MI, et al. The early detection of pancreatic cancer: what will it take to diagnose and treat curable pancreatic neoplasia? Cancer Res 2014;74:3381-9. [Crossref] [PubMed]
- Shrikhande SV, Kleeff J, Reiser C, et al. Pancreatic resection for M1 pancreatic ductal adenocarcinoma. Ann Surg Oncol 2007;14:118-27. [Crossref] [PubMed]
- Seelig SK, Burkert B, Chromik AM, et al. Pancreatic resections for advanced M1-pancreatic carcinoma: the value of synchronous metastasectomy. HPB Surg 2010;2010:579672.
- Rees M, Tekkis PP, Welsh FK, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008;247:125-35. [Crossref] [PubMed]
- Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16:1727-33. [Crossref] [PubMed]
- Tempero MA, Arnoletti JP, Behrman SW, et al. Pancreatic Adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2012;10:703-13. [Crossref] [PubMed]
- Adam R, Chiche L, Aloia T, et al. Hepatic resection for noncolorectal nonendocrine liver metastases: analysis of 1,452 patients and development of a prognostic model. Ann Surg 2006;244:524-35. [PubMed]
- Gleisner AL, Assumpcao L, Cameron JL, et al. Is resection of periampullary or pancreatic adenocarcinoma with synchronous hepatic metastasis justified? Cancer 2007;110:2484-92. [Crossref] [PubMed]
- Takada T, Yasuda H, Amano H, et al. Simultaneous hepatic resection with pancreato-duodenectomy for metastatic pancreatic head carcinoma: does it improve survival? Hepatogastroenterology 1997;44:567-73. [PubMed]
- Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg 2001;234:758-68. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [Crossref] [PubMed]
- Di Marco M, Di Cicilia R, Macchini M, et al. Metastatic pancreatic cancer: is gemcitabine still the best standard treatment? Oncol Rep 2010;23:1183-92. (Review). [Crossref] [PubMed]
- Goldstein D, El-Maraghi RH, Hammel P, et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst 2015.107. [PubMed]
- Crippa S, Bittoni A, Sebastiani E, et al. Is there a role for surgical resection in patients with pancreatic cancer with liver metastases responding to chemotherapy? Eur J Surg Oncol 2016;42:1533-9. [Crossref] [PubMed]