
Management of malignant colonic polyps
Introduction
Malignant colonic polyps (MP) are defined as sessile or pedunculated polyps that harbour cancer cells which have invaded past the muscularis mucosae into the submucosa without crossing the submucosa, regardless of lymph node involvement (1). With the implementation of colorectal cancer screening programs and the increasing availability of colonoscopy, the incidence of MP has increased (2). The prevalence of MP in endoscopically removed polyps has been reported to be between 0.75% and 5.6% (3).
Endoscopic removal of these polyps can be achieved by conventional snare polypectomy or advanced endoscopic methods such as endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) depending on the nature of the polyp and availability of expertise (4).
After a diagnosis of MP is made, the subsequent management is often contentious because the rate of remnant cancer cells in the bowel wall and regional lymph nodes vary amongst patients. Furthermore, some polyps are removed piecemeal limiting a comprehensive histological analysis of the resection margin. As a result of these variable factors, the attending surgeon is often faced with the conundrum of choosing between major oncological resection versus polypectomy alone. Major surgery though associated with morbidity and mortality, confers the benefits of clear margins, nodal harvest and allows for comprehensive disease staging which will guide the recommendation for adjuvant chemotherapy. Polypectomy saves the patient major surgery, but the issue of under-treatment with risks of recurrent and or metastatic disease remains.
Current practices are guided by the National Clinical Practice Guidelines in Oncology (NCCN) (5) and The Association of Coloproctology of Great Britain and Ireland (ACPGBI) position statement (3). This review article sets out to review the histopathologic prognostic factors of MP and the various management strategies.
Endoscopic evaluation
The management of MP begins at the index colonoscopy when a diagnosis of MP is suspected. Apart from assessing the size and shape of the polyp, the endoscopist should also evaluate its surface using the Narrow Band Imaging International Colorectal Endoscopic (NICE) (6) or Kudo (7) Classification as a guide and decide if endoscopic resection is suitable.
Sessile polyps that are NICE Type 3 or Kudo Type Vn are worrisome for deep submucosal invasion and should be considered for surgical resection instead. Pedunculated polyps on the other hand are more likely to be amenable to endoscopic resection (4). Suspected MP that are not suitable for endoscopic removal should be biopsied and their locations tattooed to facilitate subsequent surgery (3).
If not suitable for conventional snare polypectomy, the endoscopist can also consider the use of advanced endoscopic techniques such as EMR or ESD for en bloc removal of the polyp (4). Following endoscopic removal, the polypectomy site should also be tattooed to aid endoscopic re-evaluation; surgical resection (if required) and surveillance (3).
After endoscopic removal of the polyp, the patient should be assessed for risk of remnant disease and lymph node metastasis based on the following factors to determine if surgery is required or has polypectomy been adequate treatment.
Depth of invasion
The Haggitt classification is routinely used to describe the level of invasion of cancer cells in pedunculated MP and is summarized in Table 1 (8). For sessile MP, Kudo and Kikuchi described a separate classification system based on the depth of invasion into the submucosa (Table 2) (9,10). These classification systems help prognosticate and guide subsequent therapy for MP.
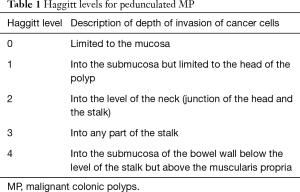
Full table

Full table
The Kikuchi classification can however be difficult to ascertain in cases where the histological specimen does not contain muscularis propria. This issue has been addressed by Kitajima et al. who described the technique of measuring the depth of submucosal invasion by cancer cells from the level of the muscularis mucosa (11).
Numerous studies demonstrate that a deeper depth of invasion is related to increased lymph node metastasis and a poorer outcome (8,10,11). Haggitt reported in 1985 that a depth of invasion to Haggitt level 4 is a significant adverse prognostic factor for pedunculated MP (8). Likewise, Nitvatvongs showed that for pedunculated MP with Haggitt levels 1 to 3, there was no incidence of lymph node metastasis but for level 4 lesions, the risk of lymph node metastasis was as high as 27% (12). Kikuchi reported that for sessile MP, the risk of lymph node metastasis was as low as 0% in SM1 lesions but increased to 14.4% in SM3 lesions (10). In 2004, Kitajima demonstrated that for sessile MP, the risk of lymph node metastasis was 0% if the depth of submucosal invasion was less than 1,000 µm but increased to more than 11.5% when the depth of invasion exceeded 1,000 µm (10). In a meta-analysis by Beaton et al., the authors also reported that the risk of lymph node metastasis was significantly higher when the depth of submucosal invasion was more than 1,000 µm (OR 3.87, 95% CI: 1.50–10.00, P=0.005) (13).
As such, surgical resection is recommended for pedunculated MP which are Haggitt level 4. For sessile MP, surgery is recommended for SM3 lesions and those with a submucosal invasion of more than 1,000 µm.
More recently, there is some evidence to show that in addition to the depth of invasion, the width and area of submucosal invasion is also a significant predictor for lymph node metastasis. Toh reported that a width of invasion more than 11.5 mm and an area of submucosal invasion more than 35 mm2 were significant predictors for lymph node metastasis (14). This study was however limited by its retrospective nature and small sample size. When more concrete data is available, these two factors may eventually impact the decision for surgery.
Lymphovascular invasion (LVI)
LVI is defined by the presence of tumour cells within an endothelial-lined channel (15). Kitajima and Okabe both demonstrated that LVI is a significant independent risk factor for lymph node metastasis (11,16). Hassan conducted a pooled-data analysis of 1,900 patients with MP and found that LVI was present in 17.6% of MP. LVI was also found to be more common in sessile MP as compared to pedunculated MP. The authors found that the rate of lymph node metastasis was significantly higher in MP with LVI than those without (35.3% vs. 7.2%, OR 7, 95% CI: 2.6–19.2). However, they did not find any significant difference in terms of residual cancer in the bowel wall, hematogenous metastasis, or mortality between the 2 groups (17). Similarly, in a meta-analysis by Beaton et al, LVI was also shown to be significantly associated with nodal involvement (OR 4.81, 95% CI: 3.14–7.37, P<0.00001) (13).
LVI is significantly associated with lymph node metastasis in MP and surgical resection is recommended.
Histological grade
There are several histological grading systems for colorectal cancer, but a single widely accepted standard is lacking (18). Furthermore, there is also significant interobserver variability amongst pathologists (3). As such the College of American Pathologists Consensus recommends a 2-tiered grading system (Table 3). By doing so, they aim to reduce the interobserver variability between pathologists and improve the prognostic significance of each grade (19).

Full table
In a pooled-data analysis by Hassan, poor differentiation was described in 7.2% and was more common in sessile MP (11.7% vs. 6.8%; P=0.05). A poor differentiation was also associated with a higher risk of lymph node metastasis (OR 3.9, 95% CI: 1.9–8.4), hematogenous metastasis (OR 3.9, 95% CI: 2–7.9) and cancer related mortality (OR 9.2, 95% CI: 4.7–18.3) (17). Likewise, Beaton reported in a meta-analysis of 13 studies that when compared to low grade tumours, high grade tumours had a higher risk of lymph node metastasis (OR 5.60, 95% CI: 2.9–10.8, P<0.00001) (13).
High grade MP are associated with a higher risk of lymph node metastasis and poorer oncological outcome and surgical resection is recommended.
Margin of resection
There is currently no consensus to the definition of a positive margin of resection (5). A positive margin has been defined as (I) tumour less than 1 mm from transected margin; (II) tumour less than 2 mm from transected margin; and (III) tumour cells within the diathermy of the transected margin (5). The Royal College of Pathologists defines a positive margin as tumour extending to ≤1 mm of the resected margin (20,21).
The literature is replete with evidence to show that if tumour cells is at or near the resection margin, there is increased adverse oncological outcomes (17,22,23,24). Hassan reported in a pooled analysis of 20 studies that the resection margin was positive in 33.2% and in these cases there was a higher residual and recurrent disease rate. (26.4% vs. 1.6%, OR 22, 95% CI: 10.3–46.6, P<0.0001). The patients with a positive resection margin also had a significantly higher rate of hematogenous metastasis and cancer related mortality (17). Similarly, Cooper described an increased rate of recurrence of up to 33% when the resection margin is ≤1 mm (23). Although there is some evidence to suggest that further treatment is only necessary when tumour is present at the true margin in the absence of other adverse histopathological features (25), it is not sufficiently strong to change the current recommendation of ≤1 mm as margin involvement (20,21).
A deep resection margin of ≤1 mm is associated with adverse oncological outcomes and surgical resection is recommended.
Tumour budding
Tumour budding is defined as a single tumour cell or a cell cluster of up to 4 tumour cells at the advancing edge of the tumour (26). Numerous studies have demonstrated an association between tumour budding and lymph node metastasis and other adverse oncological outcomes (11,22,27). In a meta-analysis of 7 studies, Beaton found that tumour budding was associated with a higher risk of lymph node metastasis (OR 7.74, 95% CI: 4.47–13.39, P<0.001) (13).
Presence of tumour budding is associated with an increased risk of lymph node metastasis and surgical resection should be considered.
Histological tumour type
MP with cribriform or micropapillary variants are associated with a higher risk of lymph node metastasis (28,29). In addition, mucinous or signet ring cell adenocarcinomas are also associated with a poorer prognosis and hence surgical resection is recommended (3,30).
Other factors
MP which are removed piecemeal often hamper accurate assessment of the resection margin and in these cases, it is prudent to offer surgical resection (3,5).
There is no consensus on the recommended imaging for staging MP. We do a staging computed tomography (CT) scan of the chest, abdomen and pelvis as a baseline at diagnosis as recommended by the ACPGBI position statement (3). If the CT shows suspicious features for lymph node metastasis, surgery is also recommended. Suspicious CT features include size >1 cm, round shape, hilar thinning, calcification, central necrosis, internal heterogeneity, irregular border or perinodal infiltration (31). The sensitivity and specificity for detecting nodal disease is however only 70% (95% CI: 63–73%) and 78% (95% CI: 73–82%) respectively as shown in a meta-analysis by Dighe et al. (32).
Surgery
If any of the high-risk factors as described above are present or if the MP is not suitable for endoscopic resection, the patient should be counselled for surgery if medically fit. The surgery should involve colectomy with en bloc removal of the regional lymph nodes (5). Laparoscopic surgery should be considered if expertise is available as this approach has been shown to be associated with shorter hospital stays and significantly faster recoveries without a difference in long term oncological outcomes (33,34).
Surveillance
The surveillance strategy would depend on whether the patient underwent surgical resection. For patients who underwent definitive oncological resection of the colon and its draining lymph nodes, the MP should be treated as a stage 1 cancer if none of the lymph nodes were involved and the surveillance strategy should be colonoscopy at 1 year and repeat in 3 years and then every 5 years if there are no advanced adenomas (5). If there are advanced adenomas at the 1-year scope, the colonoscopy should be repeated in 1 year (5). For patients with nodal involvement, they should undergo both cross sectional imaging and endoscopic surveillance along with serial carcinoembryonic antigen testing as per the NCCN guidelines (5).
There is currently no consensus for surveillance of MP treated with endoscopic resection alone. the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society recommends a surveillance scope 3 months post resection and colonoscopy at 1, 3 and 5 years post resection (35). There is a paucity of data on routine imaging surveillance for MP managed with endoscopic resection alone. The group of patients most likely to benefit from routine imaging would be the group with high risk factors for lymph node metastasis but did not undergo oncological resection. The frequency of which should be tailored according to age and co-morbidities of the patient along with the risk of developing nodal metastasis.
Conclusions
Management of MP is challenging and begins with accurate endoscopic assessment and identifying patients that are suitable for endoscopic resection. Risk of remnant disease and lymph node metastasis should then be evaluated and weighed against the risk of surgery. Patients with high risk features should undergo definitive colectomy with en bloc removal of regional lymph nodes if medically fit. Lastly, timely surveillance should detect recurrences or metachronous lesions early such that curative treatment can be instituted.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Nan Zun Teo, James Chi-Yong Ngu) for the series “Current Strategies in Colon Cancer Management” published in Journal of Gastrointestinal Oncology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo.2020.02.07). The series “Current Strategies in Colon Cancer Management” was commissioned by the editorial office without any funding or sponsorship. NZT and JCYN served as the unpaid Guest Editors of the series. The other author has no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Aarons CB, Shanmugan S, Bleier JI. Management of malignant colon polyps: current status and controversies. World J Gastroenterol 2014;20:16178-83. [Crossref] [PubMed]
- Logan RF, Patnick J, Von Wagner C, et al. English Bowel Cancer Screening Evaluation Committee. Outcomes of the Bowel Cancer Screening Programme (BCSP) in England after the first 1 million tests. Gut 2012;61:1439-46. [Crossref] [PubMed]
- Williams JG, Pullan RD, Hill J, et al. Association of Coloproctology of Great Britain and Ireland. Management of the malignant colorectal polyp: ACPGBI position statement. Colorectal Dis 2013;15:1-38. [Crossref] [PubMed]
- Rex DK, Shaukat A, Wallace MB. Optimal Management of Malignant Polyps, From Endoscopic Assessment and Resection to Decisions About Surgery. Clin Gastroenterol Hepatol 2019;17:1428-37. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology;Colon Cancer (v.3.2018). Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
- Hayashi N, Tanaka S, Hewett DG, et al. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc 2013;78:625-32. [Crossref] [PubMed]
- Kudo S, Tamura S, Watanabe H, et al. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc 1996;44:8-14. [Crossref] [PubMed]
- Haggitt RC, Glotzbach RE, Wruble LD, et al. Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy. Gastroenterology 1985;89:328-36. [Crossref] [PubMed]
- Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy 1993;25:455-61. [Crossref] [PubMed]
- Kikuchi R, Takano M, Fujiyoshi T, et al. Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum 1995;38:1286-95. [Crossref] [PubMed]
- Kitajima K, Fujimori T, Kawamata H, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol 2004;39:534-43. [Crossref] [PubMed]
- Nivatvongs S, Rojanasakul A, Jacques LF, et al. The risk of lymph node metastasis in colorectal polyps with invasive adenocarcinoma. Dis Colon Rectum 1991;34:323-8. [Crossref] [PubMed]
- Beaton C, Twine CP, Williams GL, et al. Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Colorectal Dis 2013;15:788-97. [Crossref] [PubMed]
- Toh EW, Brown P, Morris E, et al. Area of submucosal invasion and width of invasion predicts lymph node metastasis in pT1 colorectal cancers. Dis Colon Rectum 2015;58:393-400. [Crossref] [PubMed]
- Compton CC, Fielding LP, Burgart LJ, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 2000;124:979-94. [PubMed]
- Okabe S, Shia J, Nash G, et al. Lymph node metastasis in T1 adenocarcinoma of the colon and rectum. J Gastrointest Surg 2004;8:1032-9. [Crossref] [PubMed]
- Hassan C, Zullo A, Morini S, et al. Histologic risk factors and clinical outcome in colorectal malignant polyp: a pooled-data analysis. Dis Colon Rectum 2005;48:1588-96. [Crossref] [PubMed]
- Jass JR, Atkin WS, Cuzick J, et al. The grading of rectal cancer: historical perspectives and a multivariate analysis of 447 cases. Histopathology 1986;10:437-59. [Crossref] [PubMed]
- Stewart CJ, Hillery S, Plattell C. Protocol for the examination of specimens from patients with primary carcinomas of the colon and rectum. Arch Pathol Lab Med 2009;133:1359-60; author reply 1360-1. [PubMed]
- Langman G, Loughrey M, Shepherd N, et al. Association of Coloproctology of Great Britain & Ireland (ACPGBI): Guidelines for the Management of Cancer of the Colon, Rectum and Anus (2017) - Pathology Standards and Datasets. Colorectal Dis 2017;19 Suppl 1:74-81. [Crossref] [PubMed]
- Loughrey MB, Quirke B, Shepherd NA. G049 Dataset for histopathological reporting of colorectal cancer. September 2018.
- Ueno H, Mochizuki H, Hase K, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology 2004;127:385-94. [Crossref] [PubMed]
- Cooper HS, Deppisch LM, Manley PN, et al. Endoscopically removed malignant colorectal polyps: clinicopathologic correlations. Gastroenterology 1995;108:1657-65. [Crossref] [PubMed]
- Boenicke L, Fein M, Thalheimer A, et al. The concurrence of histologically positive resection margins and sessile morphology is an important risk factor for lymph node metastasis after complete endoscopic removal of malignant colorectal polyps. Int J Colorectal Dis 2010;25:433-8. [Crossref] [PubMed]
- Gill MD, Rutter MD, Holtham SJ. Management and short-term outcome of malignant colorectal polyps in the north of England. Colorectal Dis 2013;15:169-76. [Crossref] [PubMed]
- Lugli A, Kirsch R, Ajioka Y, et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol 2017;30:1299-311. [Crossref] [PubMed]
- Masaki T, Muto T. Predictive value of histology at the invasive margin in the prognosis of early invasive colorectal carcinoma. J Gastroenterol 2000;35:195-200. [Crossref] [PubMed]
- Egashira Y, Yoshida T, Takeshita A, et al. Analysis of pathological risk factors for lymph node metastasis of submucosal invasive colon cancer. Mod Pathol 2004;17:503-11. [Crossref] [PubMed]
- Xu F, Xu J, Lai M, et al. Micropapillary component in colorectal carcinoma is associated with lymph node metastasis in T1 and T2 Stages and decreased survival time in TNM stages I and II. Am J Surg Pathol 2009;33:1287-92. [Crossref] [PubMed]
- Watanabe T, Muro K, Sugihara K, et al. Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol 2018;23:1-34. [Crossref] [PubMed]
- Choi AH, Nelson RA, Arrington A, et al. Accuracy of computed tomography in nodal staging of colon cancer patients. World J Gastrointest Surg 2015;7:116-22. [Crossref] [PubMed]
- Dighe S, Purkayastha S, Brown G, et al. Diagnostic precision of CT in local staging of colon cancers: a meta-analysis. Clin Radiol 2010;65:708-19. [Crossref] [PubMed]
- Kennedy RH, Francis EA, Wharton R, et al. Multicenter randomized controlled trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme: EnROL. J Clin Oncol 2014;32:1804-11. [Crossref] [PubMed]
- Green BL, Marshall HC, Collinson F, et al. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 2013;100:75-82. [Crossref] [PubMed]
- Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology 2006;130:1872-85. [Crossref] [PubMed]


