
Magnetic resonance imaging for preoperative staging of pancreatic cancer based on the 8th edition of AJCC guidelines
Introduction
Pancreatic cancer is a malignancy that has increased in incidence in recent years, and it is the third leading cause of death among cancers (1). Radical resection is the only potentially curative treatment (2). The prognosis of patients with pancreatic cancer depends on the cancer stage at diagnosis (3). Currently, the American Joint Committee on Cancer (AJCC) TNM staging is the only indicator to evaluate the prognosis and guide clinical decision making. The size of the lesion determines the prognosis of patients with this disease; compared with larger pancreatic tumors, smaller tumors have a better prognosis (4). Previous studies have reported that the size of the tumor is a vital prognosticator, and a size-based T staging system is feasible and provides prognostic information on pancreatic cancer (5).
According to the evidence and advances in comprehending the prognosis of cancer, the TNM staging system was revised to its 8th version in 2016 (6,7). This version notably modified the T stage to be mainly dependent on tumor size rather than tumor extension beyond the pancreas; size is the best biological surrogate for pancreatic cancer after resection. The size-based staging is more reproducible and provides prognostic information (8,9). Additionally, the node-positive status was categorized into N1 and N2 stages. The T4 stage is now defined as the involvement of the celiac axis (CA) or the superior mesenteric artery (SMA), which indicates that the tumor cannot be removed. Precise preoperative staging evaluation has an important effect on determining the prognosis, and the resectability evaluation has an important effect selecting the appropriate treatment for patients with this disease. In clinical practice, among the widely used imaging tools applied to pancreatic cancer preoperative staging and resectability evaluation, magnetic resonance imaging (MRI) is commonly used due to its high soft tissue resolution, which can increase the discrimination of the cancer nidus and provide an advantage in the detection of smaller lesions (10). Dynamic contrast-enhanced MRI (DCE-MRI) makes a difference in the detection and staging of pancreatic cancer (11-14).
To our knowledge, there has been no report correlating the 8th edition of the AJCC TNM staging with preoperative MRI examinations and pathological findings. We conducted our study with the following purposes: (I) to explore the utility of MRI in the preoperative staging and resectability assessment of pancreatic cancer and (II) to compare MRI findings with pathological staging to evaluate the accuracy of MRI in the preoperative staging of pancreatic cancer based on the 8th edition of the AJCC TNM stage system.
Methods
Patient characteristics
Our study was conducted in two centers in Sichuan province. Ethical approval to perform this retrospective study was obtained from each institutional review board (IRB), and informed consent was waived. Consecutive patients with a clinical diagnosis of pancreatic cancer admitted to our institution from January 2015 to March 2019 were identified. Patients were included in our study if they met all of the following criteria: (I) patients with a histopathological diagnosis of pancreatic cancer underwent curative operation and had detailed operative records; (II) patients underwent upper abdomen MRI examination before surgery; (III) the interval time between the MRI examination and surgery was within 30 days. A total of 178 patients were recruited, and 46 patients were excluded due to chemotherapy or radiotherapy before surgery (12 patients), distant metastasis (23 patients), lesions located in the ampulla, MRI indicated biliary obstruction but no definite mass formation (6 patients) and the image quality is poor (5 patients). The final study cohort included 132 patients with pancreatic cancer. In light of the 8th edition of the AJCC TNM staging system, stage T1 was defined as a maximum tumor diameter ≤2 cm, T2 was a maximum tumor diameter >2 but ≤4 cm, T3 was a maximum tumor diameter >4 cm, and T4 was defined when the CA or the SMA was involved, indicating that the tumor could not be removed. The N stage was divided into N0 (no regional lymph node metastasis), N1 stage (metastasis in 1–3 regional lymph nodes), and N2 (metastasis in ≥4 regional lymph nodes). All the T and N stages were assessed for agreement with the MRI findings and pathological data. The condition of the CA and SMA was also evaluated; in other words, the resectability was assessed. According to National Comprehensive Cancer Network (NCCN) recommends (15,16), patients with T4 stage were borderline resectable in our study, thus surgeons performed tumor resection, dissected the CA and SMA adventitia, resected and reconstructed vascular if necessary.
MRI technique
All patients underwent a 3.0-T MR examination (MR 750, GE Medical Systems, Waukesha, WI, USA, and Achieva, Philips, the Netherlands). The general sequences consisted of T2-weighted imaging (T2WI), precontrast T1-weighted imaging (T1WI), and the arterial, portal-venous and delayed phases of DCE-MRI. For the DCE-MRI sequence, 20 mL of gadopentetate dimeglumine (Magnevist; Schering, Guangzhou, China) was administered intravenously with a pressure injector (Spectris MR Injection System, MEDRAD Inc., USA) at 2–3 mL/s, followed by a 20-mL saline solution flush. The scanning times were set to 30, 60 and 120 s after the contrast agent was injected to obtain the arterial phase, portal-venous phase and delayed phase images, respectively. Detailed information on the acquisition parameters is in Table 1.
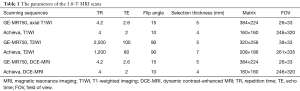
Full table
MR image interpretation
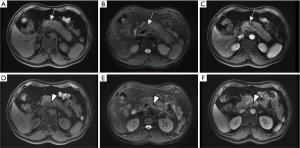
Two experienced radiologists (with at least 4 years of experience in abdominal images) processed all the MRI images independently and were blinded to the results of the intraoperative findings or pathological results. The classic appearance of pancreatic cancer on MRI is an irregular mass, hypointense on T1WI, hyperintense on T2WI and obstructing nearby ducts, either the pancreatic duct or the bile duct (13), poorly enhancing after Gadolinium enhancement (Figure 1). Two observers comprehensively analyzed all scanning sequences, including T2WI and T1WI pre- and postcontrast sequences. They independently measured the maximum diameter of the tumor in either the axial or the coronal plane and evaluated its vascular involvement, the number of regional positive lymph nodes and the distant metastasis. When their decisions were not consistent, the two observers came to a consensus by discussion. Stage T1–T3 was defined by calculating the average value of different sequences and different planes. We evaluated the invasion of the CA or SMA based on these criteria on MR images: vessel margin irregularity, periarterial fat gap disappearance, or tumor wrapping around the artery (17). A short-axis diameter of a lymph node greater than 1 cm was regarded as a lymph node metastasis (18). Integrating the TNM stage on MR images compared with intraoperative or pathological results.
To assess the reproducibility of the diameter measurement of pancreatic cancer on MRI, intraobserver reproducibility in each reader and interobserver reproducibility between the two readers were calculated separately by interclass correlation coefficients (ICCs). Two observers independently measured the maximum diameter of the tumor, and a reviewer measured them twice with an interval time of 1 week. All measurements were performed on commercially available picture archiving and communication systems (PACS) software.
Statistical analysis
The data were analyzed by using the Statistical Package for the Social Sciences (SPSS; IBM SPSS Statistics for Windows, Version 23.0, IBM Corp, Armonk, NY, USA). ICCs were used to assess the interobserver and intraobserver reproducibility for the largest diameter on T2WI, T1WI and contrast-enhanced sequences. We evaluated the consistency between MRI and pathological results in T and N staging by calculating the kappa coefficient (κ). A kappa score of 0.01–0.20 was defined as mild agreement, 0.21–0.40 fair agreement, 0.41–0.60 moderate agreement, 0.61–0.80 substantial agreement, and 0.81–0.99 almost identical agreement (19). The chi-squared test was used to assess the correlation of comprehensive TNM staging between MRI and intraoperative or histopathological results.
Results
One hundred thirty-two patients were recruited in our study, comprising 87 males and 45 females whose age ranged from 30 to 87 (mean age 58) years. In 132 patients, 132 tumors were identified. Of these, 106 tumors were pancreatic ductal adenocarcinoma, 14 tumors were mucinous adenocarcinoma, 5 tumors were adenosquamous carcinoma, and 7 tumors were acinar cell carcinoma. Regarding the locations of the tumors, 108 tumors were located in the pancreatic head and neck, and 24 tumors were located in the pancreatic body and tail. The median interval between the MRI scan and surgery was 9 (ranging from 1 to 30) days. All patients with pancreatic cancer underwent pancreatoduodenectomy or distal pancreatectomy, depending on tumor location and intraoperative findings (Table 2).
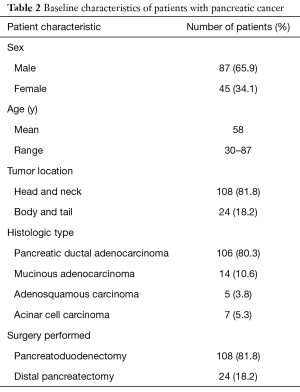
Full table
The intraobserver and interobserver ICCs for the maximum tumor diameter on T2WI were 0.922 (95% CI: 0.884–0.947) and 0.903 (95% CI: 0.857–0.904), respectively. The intraobserver and interobserver ICCs for the maximum tumor diameter on the 3D LAVA sequence before enhancement were 0.914 (95% CI: 0.893–0.944) and 0.908 (95% CI: 0.860–0.940), and after enhancement they were 0.946 (95% CI: 0.919–0.964) and 0.942 (95% CI: 0.913–0.961), respectively. This suggests that the measurement of pancreatic cancer lesions on MRI shows good reproducibility in our study.
Compared with the reference standard of intraoperative or histological results, 109 patients had a correctly assessed T stage on MRI. There were 7 cases by MRI findings for the inexact staging of T1 and T2 and MRI overestimated the tumor stage in 6 patients. The overall accuracy of assessing the T stage was 82.6% (109/132 cases). The accuracy of evaluating the T1 stage was 90% (18/20 cases), T2 was 82.4% (56/68 cases), T3 was 83.3% (15/18 cases), and T4 was 76.9% (20/26 cases). The kappa coefficient (κ=0.74) indicated substantial consistency between the MRI detection and surgical pathology for T staging (Table 3).

Full table
Overall, 98 patients with lymph node involvement were correctly evaluated by MRI (74.2% of cases). Fifteen patients showed a higher stage than shown by histopathology, and 19 patients showed a lower. The kappa coefficient (κ=0.48) indicated moderate consistency between MRI detection and pathology for N staging (Table 3).
Among the 132 patients, 26 cases were confirmed to be unresectable because of CA invasion (6 patients), SMA invasion (17 patients), or both (3 patients). Ninety-eight were correctly diagnosed as resectable tumors, and 20 were accurately diagnosed as unresectable tumors. Defining resectability as a positive case, the sensitivity was 94.2%, and the specificity was 71.4%.
All patients were comprehensively evaluated for TNM stage on MRI and histopathology, and these results are shown in Table 4. No significant difference was found between the preoperative MRI staging and histopathological staging (P=0.805).

Full table
Discussion
To the best of our knowledge, our study was the first to evaluate the overall accuracy of MRI in assessing T stage based on the 8th edition of the AJCC TNM staging system, and the accuracy of T stage performance was 82.6%. Few studies have reported the accuracy of MRI-based TNM staging. The overall accuracy of T staging was 62% in the report by Soriano et al. based on the 5th edition of the AJCC TNM staging system (20) and 56% in the report by Kauhanen et al. based on the 6th edition of the UICC TNM staging system (21). The divergence is partly due to the use of different staging systems. The significantly improved accuracy observed in our study may be associated with using the 8th edition modified T stage criteria, which mainly depend on tumor size rather than tumor extension beyond the pancreas. Quantitative assessment is more accurate, which is the reason why the accuracy of the T1–T3 stage was higher than that of the T4 stage. This suggests that MRI has a higher accuracy in evaluating T stage of pancreatic cancer according to the tumor size.
Compared with the reference standard of intraoperative findings or histopathology, the inexact staging of T1 and T2 in 7 cases by MRI findings may be due to the tumor being relatively small; thus, the errors generated during the measurement were more likely to lead to incorrect staging. The boundaries of some lesions are difficult to define on MRI, which also leads to bias in measuring the size of the tumor. MRI overestimated the tumor size in 6 patients because of peritumoral inflammation. The substantial consistency (κ=0.74) between MRI and pathology for T staging revealed that MRI is a good tool for pretreatment staging in this cohort. MRI has a higher accuracy in evaluating T stage according to the tumor size of pancreatic cancer.
MRI evaluation of regional lymph node involvement with pancreatic cancer is difficult before surgery (20). Imai et al. (22) reported the lack of accuracy of MRI on pathologic lymph node metastasis in pancreatic cancer. Several studies have reported that the accuracy of MRI for lymph node involvement ranges from 33–75% (20,22,23). Our results demonstrate that the accuracy is 74.2% for MRI preoperative assessment of lymph node status, and there is moderate consistency (κ=0.48) between MRI and pathological results for N staging, which compare favorably with the results of previous studies. No reports have previously addressed the accuracy of MRI for N staging to the N1 and N2 stages. Unfortunately, the number of cases with N2 staging is too small. This is possibly related to the fact that patients classified into the N2 stage have lost the opportunity for surgical resection. No N2-stage lymph nodes are detected on MRI, which may be associated with having too few samples. In addition, assessing lymph node metastasis only on size is not an accurate predictor of histological invasion (24). It is worth noting that only 44 patients (33.3%) have node-positive disease; increasing the number of patients may lead to an increase in the accuracy of MRI for N staging. The low detection rate by pathology may be associated with the few lymph nodes examined at our institution. Several studies have reported that the fewer the lymph nodes were detected, the higher the negative rate was (25,26). Previous reports and our results suggest that preoperative MRI is not adequate for evaluating lymph node metastasis, but it provides important preoperative information on lymph node status.
Based on the 8th edition of the AJCC TNM stage system, our evaluation of resectability depended on the invasion of the CA or SMA. Preoperative evaluation of vascular involvement plays an important role in the selection of appropriate treatment. Given this background, we assessed the role of MRI in the evaluation of artery (CA, SMA) involvement. We defined resectable tumors as positive events, and there was high sensitivity and moderate specificity. In other words, MRI has a moderate sensitivity and identical specificity in detecting artery involvement. These results were in accordance with previous reports suggesting that MRI has a low sensitivity in predicting vascular involvement (17,27). However, our results suggested that MRI can accurately evaluate the resectability of pancreatic cancer. The correlation between preoperative MRI staging and intraoperative or histopathological results was compared after we comprehensively analyzed the TNM stage of each tumor. There was no significant difference between the two (P>0.05) (Table 4). This observation implied that MRI has a certain suggestive effect on preoperative staging of pancreatic cancer. This result agrees with a previous investigation that confirmed that MRI was clinically significant for preoperative staging based on the 6th version system (28).
We evaluated intraobserver and interobserver reproducibility in the measurement of pancreatic cancer. Size is a significant parameter in size-based T staging (29) and is a vital prognosticator (5). Therefore, the measurement needs to be reproducible. Previous studies have reported that the T stage definition of pancreatic cancer in the AJCC 8th edition is reproducible (30,31). Interestingly, our study shows good intraobserver and interobserver reproducibility in the largest-diameter measurements on T1WI, T2WI and enhanced 3D LAVA.
Our study evaluated the application of MRI in the preoperative stage of pancreatic cancer. Despite the limitations of MRI in the preoperative staging, for example, the lesions were too small to be easily detected on MRI. Moreover, MRI preoperative evaluation regional lymph node involvement was difficult, previous reports (20,22) and our results suggested that preoperative MRI cannot accurately assess lymph node metastasis before surgery, whereas it provides preoperative information on lymph node status in some extent. In addition, MRI was less sensitive to vascular invasion detection (32). Nevertheless, MRI had a certain suggestive effect on the preoperative staging of pancreatic cancer.
Some limitations to our research existed. First and foremost, it was an observational and retrospective study, and the sample size was small. Prospective, multicenter and large-scale research needs to be performed to support our findings. In addition, a comparison of the diagnostic ability between CT and MRI was not performed in our study, which may limit the impact of MRI on the diagnostic value of preoperative TNM stage. Future studies should do this. Last but not least, MRI and surgical specimens were not able to directly make node-to-node comparisons; nevertheless, the lymph nodes at each site were measured and diagnosed for malignancy and these results compared with the histopathological outcomes.
Conclusions
Our results demonstrate substantial consistency between preoperative MRI and the pathological results for T staging and moderate consistency for N staging. MRI is a reliable imaging technique for the preoperative staging and resectability assessment of pancreatic cancer based on the 8th edition of the AJCC TNM staging system.
Acknowledgments
Funding: I would like to express my gratitude to all those who have helped me during the writing of my thesis. I would like to thank the teachers in our Departments of Pathology and Radiology for their help with the experiments. Last but not least, my gratitude also extends to the Applied Basic Research Program of Sichuan Province (CN), No. 2018JY0012.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo.2020.03.06). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Our retrospective study was approved by the Ethical Committee of Affiliated Hospital of North Sichuan Medical College and Deyang People’s Hospital [No. 2018ER (R) 024].
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Masiak-Segit W, Rawicz-Pruszyński K, Skórzewska M, et al. Surgical treatment of pancreatic cancer. Pol Przegl Chir 2018;90:45-53. [Crossref] [PubMed]
- Chen BB, Tien YW, Chang MC, et al. Multiparametric PET/MR imaging biomarkers are associated with overall survival in patients with pancreatic cancer. Eur J Nucl Med Mol Imaging 2018;45:1205-17. [Crossref] [PubMed]
- Zhang JW, Sun YM, Bian ZM, et al. Small pancreatic cancer diagnosis and prognosis. Zhonghua Zhong Liu Za Zhi 2009;31:375-9. [PubMed]
- Saka B, Balci S, Basturk O, et al. Pancreatic ductal adenocarcinoma is spread to the peripancreatic soft tissue in the majority of resected cases, rendering the AJCC T-stage protocol (7th edition) inapplicable and insignificant: a size-based staging system (pT1: ≤2, pT2: >2-≤4, pT3: >4 cm) is more valid and clinically relevant. Ann Surg Oncol 2016;23:2010-8.
- Chun YS, Pawlik TM, Vauthey JN. 8th edition of the AJCC cancer staging manual: pancreas and hepatobiliary cancers. Ann Surg Oncol 2018;25:845-7.
- Liu C, Cheng H, Jin K, et al. Application of the eighth edition of the American Joint Committee on Cancer staging for pancreatic adenocarcinoma. Pancreas 2018;47:742-7.
- Chen YT, Huang ZP, Zhou ZW, et al. Equipping the American Joint Committee on Cancer staging for resectable pancreatic ductal adenocarcinoma with tumor grade: a recursive partitioning analysis. Med Oncol 2016;33:122. [Crossref] [PubMed]
- Schlitter AM, Jesinghaus M, Jäger C, et al. pT but not pN stage of the 8th TNM classification significantly improves prognostication in pancreatic ductal adenocarcinoma. Eur J Cancer 2017;84:121-9. [Crossref] [PubMed]
- Borbath I, Van Beers BE, Lonneux M, et al. Preoperative assessment of pancreatic tumors using magnetic resonance imaging, endoscopic ultrasonography, positron emission tomography and laparoscopy. Pancreatology 2005;5:553-61. [Crossref] [PubMed]
- Schima W, Függer R, Schober E, et al. Diagnosis and staging of pancreatic cancer: comparison of mangafodipir trisodium-enhanced MR imaging and contrast-enhanced helical hydro-CT. AJR Am J Roentgenol 2002;179:717-24. [Crossref] [PubMed]
- Schima W, Függer R. Evaluation of focal pancreatic masses: comparison of mangafodipir-enhanced MR imaging and contrast-enhanced helical CT. Eur Radiol 2002;12:2998-3008. [Crossref] [PubMed]
- Bowman AW, Bolan CW. MRI evaluation of pancreatic ductal adenocarcinoma: diagnosis, mimics, and staging. Abdom Radiol (NY) 2019;44:936-49. [Crossref] [PubMed]
- Park HS, Lee JM, Choi HK, et al. Preoperative evaluation of pancreatic cancer: comparison of gadolinium-enhanced dynamic MRI with MR cholangiopancreatography versus MDCT. J Magn Reson Imaging 2009;30:586-95. [Crossref] [PubMed]
- Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:1028-61. [Crossref] [PubMed]
- Al-Hawary MM, Francis IR, Chari ST, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology 2014;270:248-60. [Crossref] [PubMed]
- Lee JK, Kim AY, Kim PN, et al. Prediction of vascular involvement and resectability by multidetector-row CT versus MR imaging with MR angiography in patients who underwent surgery for resection of pancreatic ductal adenocarcinoma. Eur J Radiol 2010;73:310-6. [Crossref] [PubMed]
- Lopez Hänninen E, Amthauer H, Hosten N, et al. Prospective evaluation of pancreatic tumors: accuracy of MR imaging with MR cholangiopancreatography and MR angiography. Radiology 2002;224:34-41. [Crossref] [PubMed]
- Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med 2005;37:360-3. [PubMed]
- Soriano A, Castells A, Ayuso C, et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol 2004;99:492-501. [Crossref] [PubMed]
- Kauhanen SP, Komar G, Seppänen MP, et al. A prospective diagnostic accuracy study of 18F-fluorodeoxyglucose positron emission tomography/computed tomography, multidetector row computed tomography, and magnetic resonance imaging in primary diagnosis and staging of pancreatic cancer. Ann Surg 2009;250:957-63. [Crossref] [PubMed]
- Imai H, Doi R, Kanazawa H, et al. Preoperative assessment of para-aortic lymph node metastasis in patients with pancreatic cancer. Int J Clin Oncol 2010;15:294-300. [Crossref] [PubMed]
- Kayahara M, Nagakawa T, Ohta T, et al. Analysis of paraaortic lymph node involvement in pancreatic carcinoma: a significant indication for surgery? Cancer 1999;85:583-90. [Crossref] [PubMed]
- Prenzel KL, Hölscher AH, Vallböhmer D, et al. Lymph node size and metastatic infiltration in adenocarcinoma of the pancreatic head. Eur J Surg Oncol 2010;36:993-6. [Crossref] [PubMed]
- Huebner M, Kendrick M, Reid-Lombardo KM, et al. Number of lymph nodes evaluated: prognostic value in pancreatic adenocarcinoma. J Gastrointest Surg 2012;16:920-6. [Crossref] [PubMed]
- Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol 2008;15:165-74. [Crossref] [PubMed]
- Chen FM, Ni JM, Zhang ZY, et al. Presurgical evaluation of pancreatic cancer: a comprehensive imaging comparison of CT versus MRI. AJR Am J Roentgenol 2016;206:526-35. [Crossref] [PubMed]
- Yang S, Liu J, Jin H, et al. Value of magnetic resonance images in preoperative staging and resectability assessment of pancreatic cancer. J Cancer Res Ther 2018;14:155-8. [Crossref] [PubMed]
- Kumano S, Murakami T, Kim T, et al. T staging of gastric cancer: role of multi-detector row CT. Radiology 2005;237:961-6. [Crossref] [PubMed]
- Allen PJ, Kuk D, Castillo CF, et al. Multi-institutional validation study of the American Joint Commission on Cancer (8th edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg 2017;265:185-91.
- Liu C, Cheng H, Jin K, et al. Application of the Eighth Edition of the American Joint Committee on Cancer Staging for Pancreatic Adenocarcinoma. Pancreas 2018;47:742-7.
- Horvat N, Ryan DE, LaGratta MD, et al. Imaging for pancreatic ductal adenocarcinoma. Chin Clin Oncol 2017;6:62. [Crossref] [PubMed]


