
Combined endo-laparoscopic surgery for difficult benign colorectal polyps
Introduction
Colorectal cancer (CRC) ranked globally the third for cancer incidence and fourth for cancer related death (1). CRC is developed mainly from colorectal adenomatous polyps via pathway of adenoma-carcinoma sequence and therefore, its prevention depends largely on early detection and removal of adenomatous polyps (2,3). It was reported that the incidence of CRC significantly decreased by 76–90% with endoscopic removal of colorectal adenomatous polyps (4). In recent years, there was implementation of national colorectal cancer screening program in many countries and increasing detection of premalignant or early malignant colorectal polyps was observed (5). Most of the polyps can be removed by conventional endoscopic procedures, including cold/hot biopsy, snare polypectomy or endoscopic mucosal resection (EMR). With rapid advancement in endoscopic technology, many large sessile polyps and early cancerous polyps (pTis/pT1a) can also be safely treated by endoscopic submucosal dissection (ESD) or transanal endoscopic operation (TEO) (for rectal polyps). Nonetheless, about 10–15% of polyps are considered as “difficult colorectal polyps” as they cannot be treated simply by either conventional or advanced endoscopic procedures (6), largely due to its size, morphology (sessile or flat), unfavorable location for endoscopic treatment (especially over sigmoid colon or cecum) or submucosal scar from previous attempts of endoscopic removal. These difficult colorectal polyps have historically been managed by surgical resection, which is associated with 10–15% morbidity, namely, leakage, infection and bleeding. Although minimally invasive surgery can achieve oncological safe resection for cancerous polyps, studies have reported that only a small portion (18.2–20%) of these difficult polyps are invasive cancer (7,8). Majority of the polyps can be safely treated by limited, local resection.
With the advances in imaging technology, combined endoscopic and laparoscopic surgery (CELS) have been developed to treat lesions not suitable for simple endoscopic surgery. We would like to review the current advances and clinical application of CELS in management of difficult colorectal polyps.
When colorectal polyp is found during endoscopic examination, choice of treatment is based mainly on the size of the lesion. Reported incidence of malignancy for polyps >2 cm was 18% while those <1 cm was less than 10% (9,10). Small polyps (less than 5 mm) is usually taken by cold or hot biopsy. Polyps of 6–9 mm are excised by snare polypectomy or EMR. The majority of pedunculated polyps can be resected easily by snare polypectomy. For some giant pedunculated polyps (≥30 mm), snare polypectomy can be done but is associated with higher risk of post resection bleeding. Choi et al. introduced endoscopic submucosal dissection of the polyp stalk for this kind of giant pedunculated polyps, the results were promising and en bloc resection was achieved for all patients, with no intra-operative bleeding. Post polypectomy bleeding occurred only in one case (4%), suggesting that ESD is an effective and safe option for treatment of difficult giant pedunculated or sub-pedunculated polyps (11). For sessile polyps larger than 10mm, treatment algorithm is more complicated. It is vital to distinguish malignant and benign polyps before offering endoscopic resection, as segmental colectomy should be performed for pT1b cancer, which has an incidence of approximately 10% of lymph node metastasis (12-14). The majority of benign or early cancerous polyps can be treated by endoscopic resection, like EMR or ESD. For early cancer of pTis or pT1a (submucosal invasion <1,000 mm) tumor, submucosal en bloc resection is oncological radical treatment with favorable long-time outcome. Endoscopic characteristics suggesting benign polyps are size <2 cm; soft consistency; regular contour; no depressed morphology; non-ulcerated; Kudo pit pattern type I–IV; predominant regular or sparse irregular vascular patterns on NBI; able to lift with submucosal injection.
For sessile polyps larger than 20 mm without features of malignancy, ESD should be considered as first line treatment which serves both diagnostic and therapeutic purposes. Gamaleldin et al. conducted a case-matched study comparing the outcomes of patients who underwent ESD with those who underwent laparoscopic colectomy for large benign colorectal polyps, the results showed that ESD had a 43% cost-reduction advantage over laparoscopic colectomy and a 6% lower complication rate (15). Complete resection with clear proximal, distal and circumferential margins is indispensable to enable both precise pathological diagnosis and curative potential (16). If the histology of the specimen indicates invasive carcinoma (>pT1b), a tumor-positive margin, an unfavorable histologic grade, vascular invasion, or grade 2/3 tumor budding, subsequent formal colectomy with lymph node dissection should be considered.
Despite the advances in endoscopic resection techniques for difficult colonic polyps, there is still a subgroup of polyps that are endoscopically unresectable, which has historically been managed by surgical resection. Most studies reported favorable outcomes of laparoscopic resection for endoscopically unresectable benign polyps, operative risks including iatrogenic injury, bleeding, infection, and anastomotic leakage cannot be ignored: whereas the morbidity and mortality rate were reported as 9.3–21% and 0–2.7% respectively (5,15,17).
Combining laparoscopic and endoscopic techniques to perform a local excision for difficult benign polyps is a possible alternative, and the potential risks of surgical resection is avoided. Wilhelm et al. reported a 10-year experiences and follow-up of CLES for colorectal polyps in 154 patients, showing good outcomes of CELS with a conversion rate of 5%, intraoperative and postoperative complication rate of 1% and 3%, respectively (18). There are three main kinds of CELS, which are laparoscopic-assisted colonoscopic polypectomy (LACP), full-thickness laparo-endoscopic excision (FLEX) and colonoscopy-assisted laparoscopic wedge resection (CAL-WR). The major clinical studies on CELS are listed in Table 1 (operative details) and Table 2 (surgical outcomes).
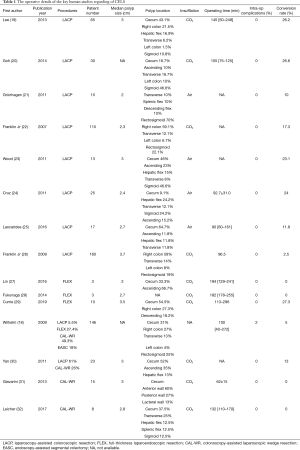
Full table
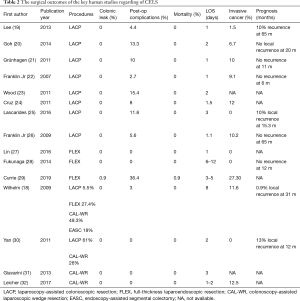
Full table
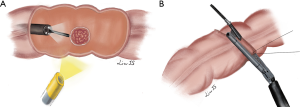
LACP (Figure 1)

This approach includes laparoscopic-assisted endoscopic EMR or ESD depending on the diameter and suspected histology of the polyps. For benign polyps less than 2 cm, laparoscopic-assisted EMR (LA-EMR) would be the preferred choice. For polyps larger than 2 cm without features of malignancy, laparoscopic-assisted piecemeal EMR or ESD (LA-ESD) can be considered. For polyps suspicious of early cancer, LA-ESD should be performed to achieved clearance of circumferential and deep margins. After induction of general anesthesia, patients will be placed in modified lithotomy position to facilitate intraoperative colonoscopy. Camera port is inserted at sub-umbilical region and pneumoperitoneum is created for laparoscopic exploration. Endoscopists then perform colonoscopy to localize the lesion and assess the suitability for EMR/ESD or need for immediate surgical resection. CO2 instead of air should be used for gas insufflation during colonoscopy (19,20), as CO2 can readily be absorbed to venous circulation. The polyp is then marked with indigo carmine (19) or vascular clip (33) intra-luminally by colonoscopy and can be identified laparoscopically. Additional 5-mm working ports (21), will be placed according to the location of the lesions (always at sites opposite to the lesions). If the polyp is located at a difficult location with sharp angulation or at retroperitoneal side, the colon is mobilized laparoscopically for better exposure and access (Figure 1A). The lesion is elevated as usual with submucosal injection of either saline (34), adrenaline in saline (20), or mannitol and methylene blue dye solution (22). EMR will be performed by endoscopic snare (Figure 1B) while ESD will be performed by use of endoscopic knifes (like Dual-knifes, Hook-knifes, IT-knifes, etc.) under direct laparoscopic vision of the colonic serosa (Figure 1C). If a full-thickness injury or perforation is suspected, laparoscopic suture repair can be performed. The integrity of the bowel can be ascertained by air-leak test with the use of CO2 insufflation using colonoscopy. The specimen is finally retrieved transanally. Some institutions perform frozen section for the resected specimens routinely. However, most believed that frozen section is unnecessary with exception for firm or multi-lobulated polyps, as the reported rate of missed cancer is low (only 1.5%) based on clinical judgement of the specimens (19).
Many authors have reported excellent outcomes of LACP compared with laparoscopic resection. The advantages of LACP are less complication, fast recovery, and less cost (24,25,35). Many centers have reported no major complication by LACP approach. Recovery is similar to endoscopic polypectomy alone, Length of stay (LOS) is 2.5 days less on average compared with laparoscopic colectomy (25,35). Lascarides et al. conducted a single-center randomized controlled study comparing LACP and laparoscopic right colectomy (LRC) for endoscopically unresectable polyps of the right colon. Results showed post-operative length of stay (LOS) was much shorter for LACP than LRC (2.63 vs. 4.94 days) (25). Long-term follow up showed lower local recurrence rate for LACP. Franklin ME Jr reported no post-operative recurrence in a mean follow-up of 63.37 months in 160 patients (26). Wilhelm et al. reported a 0.9% local recurrence rate in a mean follow up of 2.9 years (18).
LACP also is a good alternative treatment for colonic polyps (>1 cm) found under radiological examination and previously incomplete colonoscopy (obesity, redundancy of sigmoid colon, or adhesion after abdominal surgery), which accounted in 8–10% of endoscopic examinations. Quyn et al. reported successful intraoperative endoscopic polypectomy after laparoscopic colonic mobilization in all the 12 patients with incomplete preoperative colonoscopy. Recovery times were similar to endoscopic resection alone (36).
FLEX
Full-thickness resections are mandatory in some special clinical scenarios (e.g., non-lifting recurrent adenomas due to submucosal scar and adenomas located near colorectal diverticulum). The advances in endoscopic devices and techniques allow endoscopists to perform endoscopic full-thickness resection (EFTR) for these lesions. However, the safety of intraluminal closure of colonic defect is a major concern for EFTR. There are three devices designed for endoscopic defect closure in EFTR procedure with unique techniques respectively. After the colonic lesion was endoscopically marked, it was maneuvered into the resection chamber, using either traction or suction, of the full-thickness resection device (FTRD; Ovesco Endoscopy, Tubingen, Germany) (37,38). The tissue fold was then excised and a stapled anastomosis was resulted. Whereas the tissue apposition systems (TAS) (Ethicon, Endo-Surgery Inc., Cincinnati, USA) allows step-by-step closure of full-thickness colonic defect by a series of T-tags (39,40). The third device is Over-The-(endo)-Scope Clip (OTSC) (Ovesco Endoscopy, Tubingen, Germany) method, in which the edges of the colotomy were manipulated into the cap using a twin grasper (Ovesco, Endoscopy) and one or more OTSCs were deployed to close the defect (41,42). EFTR can be successfully performed in most of cases with low complication rate. Brigic et al. (43) reported a systematic review regarding the feasibility and safety of EFTR for colonic lesions, including 5 trials and 113 procedures. The result showed that overall success rate was high (89%), intraoperative complication rate was acceptable (22%). However, post-resection closure methods more commonly resulted in failure to close the defect (5–55%) and a high incidence of abnormal findings at postmortem examination (84%). EFTR should preferably be performed in high volume centers as it is indeed a technically demanding procedure associated with a steep learning curve.
Laparoscopy offer an excellent extramural view of colon and preventing collateral injuries during endoscopic treatment. Laparoscopic suture or linear stapling for closure of colotomy are relatively simple compared to intraluminal defect closure by endoscopic devices. In addition, leak test can be performed by intraluminal gas insufflation to confirm the integrity of colon after closure. Therefore, FLEX will theoretically be safer than EFTR alone for treatment of colonic lesions. Brigic et al. (44,45) successfully performed FLEX for colonic lesions in experimental pig models in 2013. There were several different modifications of FLEX reported in humans. Currie et al. (29) introduced the following method: (I) circumferential marking of resection margin of polyp using endoscopic argon plasma coagulation; (II) transmural endoscopic sutures to evert the bowel; (III) resection was completed by laparoscopic linear stapling (Figure 2). Fukunaga et al. (27,28) introduced a novel approach as: firstly, endoscopic circumferential mucosal incision was performed, then laparoscopic seromuscular dissection to meet the mucosal incision line created by the endoscopic procedure to ensure precise excision, finally linear stapler fired in an everted fashion to close the colon wall defect. For lesions located near the mesentery, Wilhelm et al. (18) suggest a small colotomy was performed after confirming location of the lesion under colonoscopic guidance, the lesion was elevated and resected by application of a linear-stapling device. Closure of the colotomy was achieved by utilizing laparoscopic sutures or linear stapling device (Figure 3). Colonic defect is closed by laparoscopic sutures or linear stapling device in the above FELX techniques. The pooled results of these small-scale case series reviewed safe closure of colonic defect without post-operative leak or abscess. There was no residual or recurrent adenoma found by colonoscopy performed in 3–12 months after resection of polyp. FELX is associated with high incidence of complete full-thickness resection of colonic polyp and successful closure of defect, with low incidence of residue lesion nor leakage.
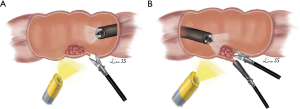
Nevertheless, accurate preoperative assessment of three morphological features of the polyp, including its relation to the mesenteric border, tumor diameter and circumferential extent of involvement of the bowel wall, has to be observed. If the lesion is located at mesenteric side or involves more than half of the circumference of colonic wall, it is considered not suitable for FLEX due to risks of bleeding, ischemia and post-operative stricture. Currie et al. (29,46) suggested computed tomography colonography (CTC) should be done to select patients for FLEX. In their study, CTC correctly identified the location of the lesions in relation to the mesenteric border in all patients. CTC was also good for the assessment of the maximum diameter of the lesion and the circumferential extent of colonic wall involvement.
CAL-WR
CAL-WR is another approach of local full-thickness resection for benign colorectal polyps with combined laparoscopic and endoscopic techniques. CAL-WR is particularly suitable for caecal polyps where the bowel wall is too thin for EMR/ESD and the size of caecum can easily accommodate a wedge resection without carrying risk of stricture. CAL-WR is technically much simpler than LACP and can be performed when LACP or FLEX failed.
As with FLEX, part of the colon, where the polyp is located, is mobilized laparoscopically to ensure tensionless during the procedure. Colonoscope is intubated to confirm location the polyp and monitor adequate surgical margins when full-thickness wall excision is performed using a linear stapler from laparoscopic side. Advancing the endoscope beyond the affected segment serves as a guidance and prevent stricture during resection (30,31). It is important to check the patency of the residual lumen by colonoscopy before stapling off the polyp. For caecal polyps, passing the colonoscope into the terminal ileum helps to protect the ileocecal valve when the stapler clamping across the cecum. The specimen will be placed in a bag and retrieved via one trocar site (Figure 4).
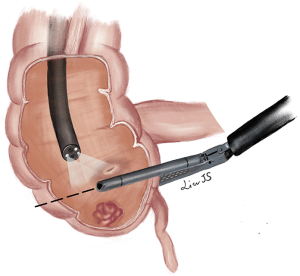
Leicher et al. (32) made a small modification of CAL-WR, which they called limited CAL-WR. After colonoscopic localization of the lesion, they placed a suture laparoscopically through the base of the polyp under endoscopic visualization. The lesion was then excised tangentially with a linear stapler with the colonic wall lifted up by applying traction on the suture. The procedure was successfully performed in 12 patients with polyps not limited to caecum but also at sigmoid, transverse colon, hepatic or splenic flexure.
CAL-WR is technically less demanding and without many complex procedures compared to LACP or FLEX. Giavarini et al. (31) and Leicher et al. (32) both reported 100% successful rate for CAL-WR in their studies. Operation time was shorter than LACP, ranging from 40–170 min. The risks for CAL-WR was much less than laparoscopic segmental colectomy (LSC). No intraoperative or postoperative complications was reported in several small-case studies. In a large cohort study including 146 patients underwent CLES and half of the patients (72 cases) underwent CAL-WR, the authors reported 2 cases of delay bleeding after operation which warranted surgical intervention, however they did not report the exact procedures performed in patients with complications (18). The hospital stay after CAL-WR was short, ranging for 1–5 days (30-32). As CAL-WR is full thickness excision, most authors had not reported any local recurrence at follow up colonoscopy. Wilhelm et al. (18) reported only one case (0.9%) out of 156 patients who had been primarily converted to open resection because of incomplete laparo-endoscopic resection found to have local recurrence, in a follow-up of 2.9 (±2.3) years.
Endoscopic-assisted laparoscopic segmental resection (EAL-SR)
For malignant polyps or those with huge diameter or located at mesenteric side, the methods mentioned above might not be suitable, and laparoscopic or open segmental resection is usually performed. Intraoperative endoscopy facilitates lesion localization and determination of extent of colonic resection. Studies have demonstrated good outcomes of this method without increasing conversion rate, operation time, or hospital stay (47,48). When the polyp is suspicious of malignancy by intraoperative colonoscopy assessment, CELS should be converted to EAL-SR for oncological radical resection.
Conclusions
With recent advancement in laparoscopic and endoscopic equipment and techniques, there are different surgical techniques for excision of benign colonic polyps without mandatory need of segmental colectomy. Combined endoscopic and laparoscopic techniques can achieved complete resection of many “difficult colonic polyps” with low intraoperative or postoperative complications. Nonetheless, there is no consensus on the indications and types of CELS procedures to be performed. Yan et al. (30) proposed a list of 15 criteria used in their center to assess the subgroup of patients who will be suitable for CELS for benign right colonic polyps, including: large or difficult anatomic location of right colonic polyps, size ≤5 cm; BMI ≤35; no emergency patients with obstruction or perforation; no IBD that needs surgery; no other abdominal malignant disease; no previous major abdominal surgeries, and so on. For benign polyps not suitable for endoscopic resection, if a patient fulfills the criteria, clinicians should consider CELS as treatment of choice before segmental colectomy. The choice of CELS largely depends on the features of the polyp, and the technical skills of surgeons and endoscopists. For polyps suspicious of invasive malignancy, segmental colectomy with lymph nodes dissection remains the preferred oncological radical treatment.
Acknowledgments
The authors thank Dr. Jing-Si Liu for her excellent figure drawings for this paper.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Nan Zun Teo, James Chi-Yong Ngu) for the series “Current Strategies in Colon Cancer Management” published in Journal of Gastrointestinal Oncology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo.2019.12.11). The series “Current Strategies in Colon Cancer Management” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, et al. The Global Burden of Cancer 2013. JAMA Oncol 2015;1:505-27. [Crossref] [PubMed]
- Sievers CK, Zou L, Pickhardt PJ, et al. Modeling the rise of intratumoral heterogeneity in growing, static, and regressing human colorectal polyps. Cancer Res Suppl 2016;12:151.
- Cho KR, Vogelstein B. Genetic alterations in the adenoma-carcinoma sequence. Cancer 1992;70:1727-31. [Crossref] [PubMed]
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993;329:1977-81. [Crossref] [PubMed]
- Marres CCM, Buskens CJ, Schriever E, et al. The impact of the national bowel screening program in the Netherlands on detection and treatment of endoscopically unresectable benign polyps Tech Coloproctol 2017;21:887-91. [Crossref] [PubMed]
- Zhang M, Shin EJ. Successful endoscopic strategies for difficult polypectomy. Curr Opin Gastroenterol 2013;29:489-894. [PubMed]
- Ross HM, Li C, Rosenthal J, et al. Laparoscopic colon resection for polyps: a good novice case? Dis Colon Rectum 2006;49:879-82. [Crossref] [PubMed]
- Pokala N, Delaney CP, Kiran RP, et al. Outcome of laparoscopic colectomy for polyps not suitable for endoscopic resection Surg Endosc 2007;21:400-3. [Crossref] [PubMed]
- Kudo S, Kashida H, Tamura T, et al. Colonoscopic diagnosis and management of nonpolypoid early colorectal cancer. World J Surg 2000;24:1081-90. [Crossref] [PubMed]
- Bertelson NL, Kalkbrenner KA, Merchea A. Colectomy for endoscopically unresectable polyps: how often is it cancer? Dis Colon Rectum 2012;55:1111-6. [Crossref] [PubMed]
- Choi YS, Lee JB, Lee EJ, et al. Can endoscopic submucosal dissection technique be an alternative treatment option for a difficult giant (≥ 30 mm) pedunculated colorectal polyp? Dis Colon Rectum 2013;56:660-6. [Crossref] [PubMed]
- Nakadoi K, Tanaka S, Kanao H, et al. Management of T1 colorectal carcinoma with special reference to criteria for curative endoscopic resection. J Gastroenterol Hepatol 2012;27:1057-62. [Crossref] [PubMed]
- Suh JH, Han KS, Kim BC, et al. Predictors for lymph node metastasis in T1 colorectal cancer. Endoscopy 2012;44:590-5. [Crossref] [PubMed]
- Son HJ, Song SY, Lee WY, et al. Characteristics of early colorectal carcinoma with lymph node metastatic disease. Hepatogastroenterology 2008;55:1293-7. [PubMed]
- Gamaleldin M, Benlice C, Delaney CP, et al. Management of the colorectal polyp referred for resection: A case-matched comparison of advanced endoscopic surgery and laparoscopic colectomy. Surgery 2018;163:522-7. [Crossref] [PubMed]
- Watanabe T, Itabashi M, Shimada Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int J Clin Oncol 2015;20:207-39. [Crossref] [PubMed]
- Hauenschild L, Bader FG, Laubert T, et al. Laparoscopic colorectal resection for benign polyps not suitable for endoscopic polypectomy. Int J Colorectal Dis 2009;24:755-9. [Crossref] [PubMed]
- Wilhelm D, von Delius S, Weber L, et al. Combined laparoscopic-endoscopic resections of colorectal polyps:10-year experience and follow-up. Surg Endosc 2009;23:688-93. [Crossref] [PubMed]
- Lee SW, Garrett KA, Shin JH, et al. Dynamic article: Long-term outcomes of patients undergoing combined endolaparoscopic surgery. Dis Colon Rectum 2013;56:869-73. [Crossref] [PubMed]
- Goh C, Burke JP, McNamara DA, et al. Endolaparoscopic removal of colonic polyps. Colorectal Dis 2014;16:271-5. [Crossref] [PubMed]
- Grünhagen DJ, van Ierland MC, Doornebosch PG, et al. Laparoscopic-monitored colonoscopic polypectomy: a multimodality method to avoid segmental colon resection. Colorectal Dis 2011;13:1280-4. [Crossref] [PubMed]
- Franklin ME Jr, Leyva-Alvizo A, Abrego-Medina D, et al. Laparoscopically monitored colonoscopic polypectomy: an established form of endoluminal therapy for colorectal polyps. Surg Endosc 2007;21:1650-3. [Crossref] [PubMed]
- Wood JJ, Lord AC, Wheeler JM, et al. Laparo-endoscopic resection for extensive and inaccessible colorectal polyps: a feasible and safe procedure. Ann R Coll Surg Engl 2011;93:241-5. [Crossref] [PubMed]
- Cruz RA, Ragupathi M, Pedraza R, et al. Minimally Invasive Approaches for the Management of “Difficult” Colonic Polyps. Diagn Ther Endosc 2011;2011:682793.
- Lascarides C, Buscaglia JM, Denoya PI, et al. Laparoscopic right colectomy vs laparoscopic-assisted colonoscopic polypectomy for endoscopically unresectable polyps: a randomized controlled trial. Colorectal Dis 2016;18:1050-6. [Crossref] [PubMed]
- Franklin ME Jr, Portillo G. Laparoscopic monitored colonoscopic polypectomy: long-term follow-up World J Surg 2009;33:1306-9. [Crossref] [PubMed]
- Lin AY, O'Mahoney PR, Milsom JW, et al. Dynamic Article: Full-Thickness Excision for Benign Colon Polyps Using Combined Endoscopic Laparoscopic Surgery. Dis Colon Rectum 2016;59:16-21. [Crossref] [PubMed]
- Fukunaga Y, Tamegai Y, Chino A, et al. New Technique of En Bloc Resection of Colorectal Tumor Using Laparoscopy and Endoscopy Cooperatively (Laparoscopy and Endoscopy Cooperative Surgery - Colorectal) Dis Colon Rectum 2014;57:267-71. [Crossref] [PubMed]
- Currie AC, Blazeby JM, Suzuki N, et al. Evaluation of an early-stage innovation for full-thickness excision of benign colonic polyps using the IDEAL framework. Colorectal Dis 2019;21:1004-16. [Crossref] [PubMed]
- Yan J, Trencheva K, Lee SW, et al. Treatment for right colon polyps not removable using standard colonoscopy: combined laparoscopic-colonoscopic approach. Dis Colon Rectum 2011;54:753-8. [Crossref] [PubMed]
- Giavarini L, Boni L, Cortellezzi CC, et al. Laparoscopic caecal wedge resection with intraoperative endoscopic assistance. Int J Surg 2013;11 Suppl 1:S58-60. [Crossref] [PubMed]
- Leicher LW, de Vos Tot Nederveen Cappel WH, van Westreenen HL. Limited Endoscopic-Assisted Wedge Resection for Excision of Colon Polyps. Dis Colon Rectum 2017;60:299-302. [Crossref] [PubMed]
- Hensman C, Luck AJ, Hewett PJ, et al. Laparoscopic-assisted colonoscopic polypectomy: technique and preliminary experience. Surg Endosc 1999;13:231-2. [Crossref] [PubMed]
- Lee MK, Chen F, Esrailian E, et al. Combined endoscopic and laparoscopic surgery may be an alternative to bowel resection for the management of colon polyps not removable by standard colonoscopy. Surg Endosc 2013;27:2082-6. [Crossref] [PubMed]
- Prohm P, Weber J, Bönner C, et al. Laparoscopic-assisted coloscopic polypectomy Dis Colon Rectum 2001;44:746-8. [Crossref] [PubMed]
- Quyn AJ, Vujovic Z, Ziyaie D, et al. Laparoscopic-assisted colonoscopy: results and follow-up endoscopic success. Colorectal Dis 2016;18:O376-79. [Crossref] [PubMed]
- Velegraki M, Trikola A, Vasiliadis K, et al. Endoscopic full-thickness resection of colorectal lesions with the full-thickness resection device: clinical experience from two referral centers in Greece Ann Gastroenterol 2019;32:482-8. [PubMed]
- Kuellmer A, Mueller J, Caca K, et al. Endoscopic full-thickness resection for early colorectal cancer. Gastrointest Endosc 2019;89:1180-9.e1. [Crossref] [PubMed]
- Raju GS, Malhotra A, Ahmed I. Colonoscopic full-thickness resection of the colon in a porcine model as a prelude to endoscopic surgery of difficult colon polyps: a novel technique (with videos). Gastrointest Endosc 2009;70:159-65. [Crossref] [PubMed]
- Delaney CP, Champagne BJ, Marks JM, et al. Tissue apposition system: new technology to minimize surgery for endoscopically unresectable colonic polyps. Surg Endosc 2010;24:3113-8. [Crossref] [PubMed]
- Schmidt A, Beyna T, Schumacher B, et al. Colonoscopic full-thickness resection using an over-the-scope device: a prospective multicentre study in various indications Gut 2018;67:1280-9. [Crossref] [PubMed]
- von Renteln D, Kratt T, Rosch T, et al. Endoscopic full-thickness resection in the colon by using a clip-and-cut technique: an animal study. Gastrointest Endosc 2011;74:1108-14. [Crossref] [PubMed]
- Brigic A, Symons NR, Faiz O, et al. A systematic review regarding the feasibility and safety of endoscopic full thickness resection (EFTR) for colonic lesions. Surg Endosc 2013;27:3520-9. [Crossref] [PubMed]
- Brigic A, Southgate A, Sibbons P, et al. Full-thickness laparoendoscopic colonic excision in an experimental model Br J Surg 2013;100:1649-54. [Crossref] [PubMed]
- Brigic A, Southgate A, Sibbons PD, et al. Full-thickness laparoendoscopic stapled excision of colonic lesion in a porcine ex vivo model. Endoscopy 2013;45 Suppl 2 UCTN:E167-8.
- Currie AC, Burling D, Mainta E, et al. An analysis of the accuracy of computed tomography colonography when defining anatomy for novel full-thickness colonic excision techniques in early colonic neoplasia. Colorectal Dis 2016;18:983-88. [Crossref] [PubMed]
- Zmora O, Dinnewitzer AJ, Pikarsky AJ, et al. Intraoperative endoscopy in laparoscopic colectomy. Surg Endosc 2002;16:808-11. [Crossref] [PubMed]
- Gorgun IE, Aytac E, Manilich E, et al. Intraoperative colonoscopy does not worsen the outcomes of laparoscopic colorectal surgery: a case-matched study. Surg Endosc 2013;27:3572-6. [Crossref] [PubMed]


