Liver-first approach to stage IV colon cancer with synchronous isolated liver metastases
Introduction
Colon cancer remains one of the most common malignancies in the United States with 97,220 new cases expected in 2018 (1). Distant metastases account for 90% of deaths, with only 12% of patients surviving beyond 5-years after diagnosis with stage IV disease (1). Approximately 1/3 of patients with metastatic colon cancer present with isolated liver metastases (ILM) (2). A subset of ILM are amenable to treatment with liver resection or liver directed therapies equating to improved survival (3-6).
To treat ILM arising from colon cancer, some surgeons favor a staged surgical approach entailing primary tumor resection (colectomy) followed by eradication of remaining metastases with hepatectomy and/or locoregional therapy (7,8). Others simultaneously resect primary and metastatic tumors in the same operative setting (9-11). A third strategy, called the liver-first approach (LFA), focuses on elimination of metastatic disease with staged colectomy (12). Level 1 data has not been collected to support one sequence over another. Furthermore, scarce retrospective data exists to evaluate these approaches, specifically in colon (excluding rectal) adenocarcinoma (CAC).
This study queries the National Cancer Data Base (NCDB) to examine treatment practices in the United States for the surgical management of CAC presenting with synchronous ILM. A secondary objective is to examine if a particular sequence of surgeries to the primary tumor and metastases equates to improved clinical outcome.
Methods
Data source
The NCDB is a tumor registry curated by the Commission on Cancer of the American College of Surgeons and the American Cancer Society capturing facility-based oncology data from approximately 70% of all newly diagnosed malignancies in the United States (13). The 2015 edition of the Participant User File was used for this analysis in accordance with our Institutional Review Board approved protocol with patient consent waived. According to NCDB policy: “the data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.” (14). The NCDB provides data on patient characteristics, tumor staging, treatment and survival. A complete list of the variables provided in the NCDB can be accessed online (14).
Patient selection
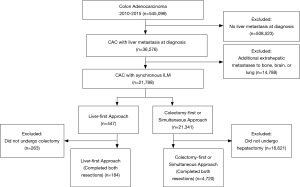
All adult patients (age >20) diagnosed with CAC with liver metastases from 2010–2015 were identified. Given the distinct biology and treatment of rectal cancer (RC), we excluded patients with RC to avoid confounding effects. Patients with additionally documented lung, bone or brain metastases were excluded (Figure 1). Data on metastases to other anatomic sites is not available in the NCDB. Patients were defined as having a metastasectomy if the NCDB variable “surgery other site” was recorded as, “nonprimary surgical procedure to distant site.” (15). Patients who did not undergo either metastasectomy or definitive resection of their primary were excluded. Timing of resection was determined using the NCDB variables “first surgical procedure, days from diagnosis” and “definitive surgical procedure, days from diagnosis”. Of those patients, treatment cohorts were stratified according to the sequence of resection approach with an intention-to-treat paradigm: liver-first (n=447), and colectomy-first/synchronous (n=21,341) (16). Patients were defined as intent to treat based on initial operation performed, regardless if they completed resection of the remaining tumor burden (17,18).
Statistical analysis
Characteristics between the groups were compared using Chi-square test and Student’s t test. Survival from time of diagnosis was compared with Kaplan-Meier analysis in the subset of patients with known follow up data (n=358 liver-first, n=18,042 colectomy-first/simultaneous). In an effort to determine variables that may confound or explain the differences in survival between the groups, two Cox proportional hazards analyses were constructed. The first model was adjusted for patient and tumor characteristics including age, sex, race, Charlson Deyo comorbidity index score, tumor size, lymph node metastases and year of diagnosis. The next model additionally included concomitant treatment with chemotherapy prior to and after surgery.
Results
Patient, tumor and treatment characteristics
A total of 545,099 patients diagnosed with CAC from 2010–2015 were identified. Seven percent (n=36,576) had hepatic metastases at diagnosis without extrahepatic metastases to the bone, brain or lung. Of these patients, the majority (21,788, 60%) underwent surgical intervention (Figure 1). The LFA was employed in only 2% (n=447) of cases, but was associated with a higher rate of completion resection of the remaining tumor (subsequent colectomy) compared to patients who underwent colectomy first or simultaneous resection (via subsequent hepatectomy) (41% vs. 22%, P<0.001, respectively).
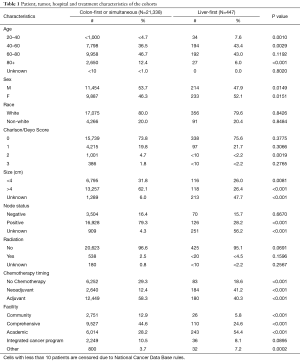
Patients who underwent the LFA were similar to those undergoing the colectomy first or simultaneous approach in terms of sex, race, and treatment with radiation. However, patients who were selected for the LFA were younger, less comorbid (by Charlson Comorbidity score) and more commonly received chemotherapy (P<0.05) (Table 1). Strikingly, the proportion of patients who had received upfront chemotherapy was markedly larger for the liver-first group (41.2% vs. 12.4%, P<0.001).
Full table
Hospital characteristics
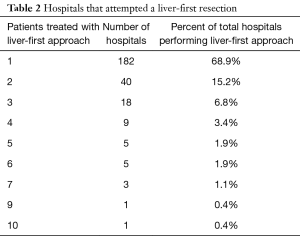
Many hospitals attempted at least one LFA resection but few had repeated experience during the study period. Out of 1,323 hospitals that treated patients with colon cancer with hepatic metastasis in the NCDB, 649 (49%) attempted a liver-first resection. Notably, 84% of the hospitals that attempted the LFA only did so for 1–2 cases during the study period (Table 2). Patients undergoing the LFA were also more likely to be treated at an academic center compared to a community or comprehensive cancer center (54.4% vs. 5.8%, 54.4% vs. 24.6%, P<0.001) (Table 1).
Full table
Survival
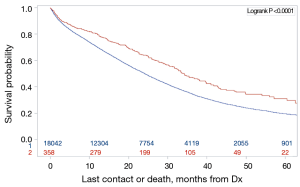
Kaplan Meier analysis demonstrated increased survival associated with a LFA compared to the colectomy-first and simultaneous resection group [median survivals: 34 months, 95% CI (30.5–39.6 months) vs. 24 months, 95% CI (23.7–24.6 months); logrank P<0.0001] (Figure 2).
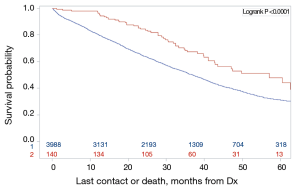
To evaluate if these findings seen in the intention-to-treat groups were attributable to a higher rate of subsequent complete resection, we restricted analysis to only patients who had complete resections of their primary tumor and metastases (n=140 liver-first, n=3,988 colectomy-first or simultaneous). In this subset of patients, the liver-first group continued to have a longer survival [median survivals: 57 months; 95% CI (42.7–73.2 months) vs. 36 months; 95% CI (35.4–38.4 months), logrank P<0.001] (Figure 3).
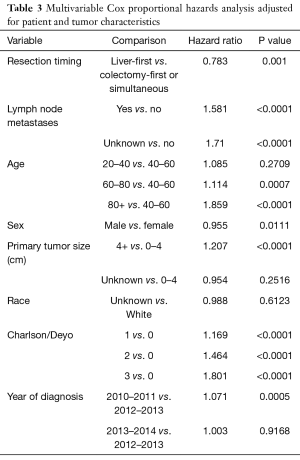
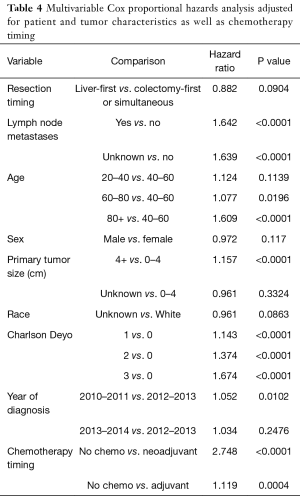
To account for confounding, a Cox proportional hazards model adjusted for patient and tumor characteristics was constructed, which also demonstrated decreased risk of death for patients with the LFA [HR 0.783; 95% CI (0.67–0.89), P=0.001]. (Table 3). To investigate the impact of different systemic therapy approaches, additional adjustment for utilization and timing (post-resection/pre-resection) of chemotherapy treatment was sequentially added to the model. The survival trend between approaches still favored the LFA [HR 0.882; 95% CI (0.75–1.01) P=0.0904] (Table 4).
Full table
Full table
Conclusions
The only possibility for cure in patients with CAC with ILMs is resection of both the primary and metastatic tumors (10). Thus, optimization of surgical approach is paramount. Our study provides the first analysis of the LFA to managing CAC with ILMs, excluding RC due to its differing tumor biology and multimodality treatment strategy. Using the NCDB we identified only a very small proportion of patients (2%) in the United States who present with CAC with synchronous ILMs who are treated with an LFA. This approach, in contradistinction to synchronous resections and a colectomy-first approach, is associated with an improved median survival of 34 vs. 24 months (P<0.001) in all patients and 57 vs. 36 months (P<0.001) for patients completing resection of the primary and metastatic tumors. Further, patients treated with this approach are more likely to complete resection of metastatic and primary tumors, receive upfront chemotherapy and more likely to be treated at academic centers. Prior to our study, the limited knowledge about outcomes of LFA in CAC patients was largely extrapolated from RC cohorts (12,19-22).
Early adopters of the liver-first technique have reported mostly RC outcomes, with 3-year OS ranging from 41–89% in small retrospective cohorts (23). The largest meta-analysis to date, reviewing outcomes for 133 patients treated with a LFA, did not find evidence suggesting sequence of resection contributes to difference in outcomes (24,25). Compared to historical data, the liver-first outcome of 48% 5-year OS in patients with CAC surpasses historical controls.
Although survival was still clearly improved for patients who received the LFA after adjusting for patient and tumor characteristics, this effect was mitigated by further adjustment for chemotherapy approach. More specifically, after adjusting for the effect and timing of chemotherapy, the LFA trended towards a more favorable outcome but was no longer significant. The EORTC (Nordlinger et al.) randomized trial showed no improvement in OS but did show improvement in DFS in a perioperative chemotherapy approach for liver metastasectomy, after previous staged resection of the primary tumor (10). A possible explanation is that while upfront chemotherapy may not itself improve overall survival, it may allow time to select patients with favorable biology for surgery, while those with unfavorable biology drop-out. This factor may also contribute to reports of up to 35% of patients failing to complete intended resection of both primary and metastatic tumors irrespective of surgical strategy (26). Our study highlights the heterogeneous use of upfront chemotherapy to treat CAC with ILM, with liver-first patients having a higher utilization. This is consistent with prior report of preferential utilization of neoadjuvant chemotherapy in patients receiving an LFA (25). It is unknown whether the LFA in itself facilitates that greater use of chemotherapy or otherwise.
When employing a staged resection approach, complete of resection of both primary and metastatic disease is a key treatment goal. Despite being used in only 2% of cases, the LFA enjoyed a relatively high (41.2%) rate of completion colectomy. The completion rate in this study is likely an underestimate, as the NCDB likely did not completely capture all follow-up data on patients who underwent LFA shortly prior to end of the study period. Previous studies reporting RC outcomes have demonstrated completion rates approaching 85% in carefully selected patients (19).
This analysis identified that there is indeed selection bias among the patients who were selected for the LFA. They were more likely to be younger, less comorbid and seen at academic centers. For example, 37% of patients in the colectomy-first/simultaneous resection group were 40–60 years of age, compared to 43% in the liver-first group (P=0.003). A core principle of personalized surgical oncology is to select patients who will do well based on their demographics and tumor biology (27). Our data suggests that patients who have been selected for this approach have favorable long-term survival outcomes.
We note several limitations of our study. We were unable to separate patients who were treated with a colectomy-first approach and patients treated with simultaneous resection due to the nature of the NCDB variables. By combining these two groups we lose the ability to understand each method’s contribution to clinical outcomes. However, prior comparison between classical approach and combined approach yielded no difference in outcomes between the approaches (9). Due to the nature of a large retrospective clinical database, we were unable to evaluate several key data items, such as size and number of liver metastases, hepatectomy-specific variables, such as R0 resection status, minor or major resection, and utilization of open or laparoscopic approach. Going forward, collection of these data points in registry data would help interpretation of our findings.
Current recommendations for symptomatic CAC with resectable synchronous ILMs are for staged resection of perforated or occlusive primary tumors, followed by chemotherapy and subsequent metastasectomy (28). This likely increases the representation of patients presenting with symptomatic disease in the colon-first/combined resection cohort, potentially influencing outcomes. NCDB data does not account for primary tumor symptomatology, and our results must be interpreted in the context of the liver-first group having potentially more favorable disease.
The mandate of personalizing cancer care is reliant on genomics to guide clinical decision making to select patients for optimal outcomes. While evidence mounts to personalized care reliant on genomics, we must continue to use clinical and pathological parameters to tailor complex treatment decisions in cancer (29). We show that patients who present with synchronous colon cancer with liver metastases are treated with multiple treatment approaches. In the United States, utilization of a LFA is not commonly employed, yet may lead to improved clinical outcomes. Our study underscores that careful surgical planning may lead to long term survival in patients with colon cancer liver metastases.
Acknowledgments
Funding: This publication was made possible by CTSA Grant Number UL1 TR001863 from the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. This work was also supported by the Lampman Research Fund in Yale Surgical Oncology.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- American Cancer Society. Cancer Facts & Figures. 2018. Atlanta: American Cancer Society, 2018.
- Siegel RL, Miller KD, Jemal A. Cancer statistics 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18. [Crossref] [PubMed]
- White RR, Avital I, Sofocleous CT, et al. Rates and patterns of recurrence for percutaneous radiofrequency ablation and open wedge resection for solitary colorectal liver metastasis. J Gastrointest Surg 2007;11:256-63. [Crossref] [PubMed]
- Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 2006;141:460-6. [Crossref] [PubMed]
- Simmonds PC, Primrose JN, Colquitt JL, et al. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer 2006;94:982. [Crossref] [PubMed]
- Nordlinger B, Quilichini MA, Parc R, et al. Surgical resection of liver metastases from colo-rectal cancers. Int Surg 1987;72:70-72. [PubMed]
- Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol 1997;15:938-46. [Crossref] [PubMed]
- Silberhumer GR, Paty PB, Denton B, et al. Long-term oncologic outcomes for simultaneous resection of synchronous metastatic liver and primary colorectal cancer. Surgery 2016;160:67-73. [Crossref] [PubMed]
- Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 1996;77:1254-62. [Crossref] [PubMed]
- Martin R, Paty P, Fong Y, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver cancer. J Am Coll Surg 2003;197:233-41. [Crossref] [PubMed]
- Mentha G, Majno PE, Andres A, et al. Neoadjuvant chemotherapy and resection of advanced synchronous liver metastases before treatment of the colorectal primary. Br J Surg 2006;93:872-8. [Crossref] [PubMed]
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol 2017;3:1722-8. [Crossref] [PubMed]
- National Cancer Database. American College of Surgeons. Available online: https://www.facs.org/quality-programs/cancer/ncdb. Accessed August 30, 2018.
- Krell RW, Regenbogen SE, Wong SL. Variation in hospital treatment patterns for metastatic colorectal cancer. Cancer 2015;121:1755-61. [Crossref] [PubMed]
- Greenleaf EK, Sun SX, Hollenbeak CS, et al. Minimally invasive surgery for gastric cancer: the American experience. Gastric Cancer 2017;20:368-78. [Crossref] [PubMed]
- Hernán MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology 2008;19:766-79. [Crossref] [PubMed]
- Gupta SK. Intention-to-treat concept: A review. Perspect Clin Res 2011;2:109-12. [Crossref] [PubMed]
- Verhoef C, van der Pool AE, Nuyttens JJ, et al. The “liver-first approach” for patients with locally advanced rectal cancer and synchronous liver metastases. Dis Colon Rectum 2009;52:23-30. [Crossref] [PubMed]
- Buchs NC, Ris F, Majno PE, et al. Rectal outcomes after a liver-first treatment of patients with stage IV rectal cancer. Ann Surg Oncol 2015;22:931-7. [Crossref] [PubMed]
- Charnsangavej C, Clary B, Fong Y, et al. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol 2006;13:1261-8. [Crossref] [PubMed]
- Zarour LR, Anand S, Billingsley KG, et al. Colorectal Cancer Liver Metastasis: Evolving Paradigms and Future Directions. Cell Mol Gastroenterol Hepatol 2017;3:163-73. [Crossref] [PubMed]
- Lam VW, Laurence JM, Pang T, et al. A systematic review of a liver-first approach in patients with colorectal cancer and synchronous colorectal liver metastases. HPB (Oxford) 2014;16:101-8. [Crossref] [PubMed]
- Kelly ME, Spolverato G, Lê GN, et al. Synchronous colorectal liver metastasis: a network meta-analysis review comparing classical, combined, and liver-first surgical strategies. J Surg Oncol 2015;111:341-51. [Crossref] [PubMed]
- Valdimarsson VT, Syk I, Lindell G, et al. Outcomes of liver-first strategy and classical strategy for synchronous colorectal liver metastases in Sweden. HPB (Oxford) 2018;20:441-7. [Crossref] [PubMed]
- Sturesson C, Valdimarsson VT, Blomstrand E, et al. Liver-first strategy for synchronous colorectal liver metastases – an intention-to-treat analysis. HPB (Oxford) 2017;19:52-8. [Crossref] [PubMed]
- Roukos DH. Cancer genome explosion and systems biology: impact on surgical oncology? Ann Surg Oncol 2011;18:12-5. [Crossref] [PubMed]
- Adam R, de Gramont A, Figueras J, et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev 2015;41:729-41. [Crossref] [PubMed]
- Pitroda SP, Khodarev NN, Huang L, et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat Commun 2018;9:1793. [Crossref] [PubMed]