Postoperative chemoradiotherapy vs. preoperative chemoradiotherapy for locally advanced (operable) gastric cancer: clarifying the role and technique of radiotherapy
Introduction
Worldwide, almost one million new cases of stomach cancer were estimated to have occurred in 2012, making it the fifth most common cancer, and the third leading causing of cancer deaths (1). The 7th edition of the tumor node metastasis (TNM) staging system, revised based on the evidence that exists around prognostic factors and current treatment strategies, emphasizes the importance of depth of invasion and the number of locoregional nodes involved as major prognostic factors. For the first time, this represents a consensus approach of Eastern and Western countries (2,3). To facilitate reporting and provide guidance for patients with gastroesophageal (GE) junction cancers, they are now classified under esophageal cancer, although it is important to remind ourselves that many clinical trials designed for gastric cancers include a significant proportion of GE junction tumors and many esophageal cancer trials also included some proximal gastric cancers, complicating the interpretation of the literature and its application in clinical practice.
There are many heterogeneous subgroups under the broad heading of gastric cancers. Tumors arising from different anatomical locations have access to different routes of spread. Tumors with different histological (e.g., diffuse vs. others) and molecular [e.g., human epidermal growth factor receptor-2 (HER2)] (4) characteristics have different etiology (5), prognosis (4,6), and response to therapy (7). Patients from Asia, North America and Europe differ in terms of their toxicity profiles and response to treatments (8).
The objective of this review is to provide the rationale, evidence and technical considerations comparing the use of pre and postoperative radiotherapy (RT) in gastric cancer.
What is locally advanced gastric cancer?
While what constitutes early gastric cancer is relatively well defined (9), there is considerable variability in what is considered locally advanced disease. DE Sol et al. (10) provided a summary of definitions extracted from recent trials highlighting this variation. A minority of authors use the term to describe the locoregional extent of disease irrespective of whether distant disease is present, while the more common approach refers to patients with no evidence of metastatic disease (M0), where invasion of muscularis and beyond is present, with or without nodal involvement. For example, the pivotal randomized trial reported by Macdonald et al. (11) in the management of gastric cancer that resulted in the generalized adoption of postoperative chemoradiotherapy (CRT) employs the definition of stage Ib-IV (M0) as advanced cancers.
For the majority of investigators, the term locally advanced gastric cancer is a general term that is used to describe patients with a modest survival with surgery alone. For the purpose of this review, we will focus our deliberations with this definition in mind, where the risk of recurrence would justify the use of adjuvant or neoadjuvant therapies. In TNM terms, patients with locoregional disease, with T stage of submucosal involvement or higher or node positive disease (T2-4, N1-3, M0; TNM v7) are being staged as locally advanced gastric cancer, with a five-year overall survival (OS) rate following complete resection in the range of 57% (3).
Anatomical definition of lymph node stations for gastric cancer was described by the Japanese Gastric Cancer Association and has been widely adopted. Nodal stations 1-12 (1: paracardial nodes to 12: hepatoduodenal ligament nodes) and 14v (lymph nodes along inferior mesenteric vein) are defined as regional gastric lymph nodes, while metastasis to any other nodes are classified as M1 (12). While the prognostic value of the number of involved nodes is of critical importance, the anatomic extent of metastatic nodes also conveys prognostic significance, with extra-perigastric nodal stations conveying a worse prognosis than the perigastric nodes (13,14).
What is optimal surgery?
A discussion on the role of neoadjuvant or adjuvant therapy is incomplete without a brief consideration of the clinical impact of the type and extent of surgery, the central curative modality for patients with gastric cancer. While the fundamental surgical principles of achieving a complete resection with negative margins, and the more recently adopted quality indicator of a minimum of nodes resected (e.g., 16) (15) are uniformly accepted, significant variations in approach exist in other areas of surgical decision-making.
The extent of gastric resection is based on oncologic principles. The location, extent and type of gastric cancer will dictate the extent of resection. Diffuse type cancers require a total gastrectomy, regardless of the location of the gross tumor. Total gastrectomy is required for large tumors or tumors of the lesser curve or body of the stomach. Antral cancers may be adequately resected with a distal gastrectomy if a 5 cm margin can be achieved. Proximal gastric cancers are generally resected by a total gastrectomy because of the poor functional result due to intractable reflux esophagitis when the distal stomach is anastomosed to the esophagus. Locally advanced proximal cancers often require resection of the spleen and tail of pancreas because of direct extension of the primary tumor. If the tumor involves the distal esophagus, a 5 cm margin or more on the esophagus is required to reduce anastomotic recurrences. Rarely, a proximal gastrectomy with reconstruction using a short segment of pedicle jejunum is used for small tumors of the proximal stomach, allowing preservation of the antrum.
The major factor of ongoing debate is the extent of lymph node dissection. D1 dissection generally describes the removal of nodal stations 1-7 (perigastric nodes including pericardial, lesser curvature, greater curvature, supra and infrapyloric, along the trunk of L gastric artery) while D2 dissection refers to the removal of lymph node stations up to 12 (D1 and splenic hilar, hepatoduodenal ligament) (12). The effect of an extended lymphadenectomy provides greater clearance of locoregional nodes and potentially better sampling of the nodes. Extended vs. limited (D2 vs. D1) dissections were compared in several randomized trials and summarized most recently using a systematic review by Jiang et al. (16). Data from eight randomized trials conducted in Asia, Europe and Africa involving over 2,000 patients were included. Five-year OS was similar between the two approaches. However, postoperative mortality rates were significantly higher for patients treated with D2 dissection [D2 vs. D1, 18% vs. 11%; relative risk (RR) 0.58, 95% confidence interval (CI): 0.47-0.71]. Other morbidities (e.g., anastomotic leak, pancreatic leak, reoperation rates, wound infection, pulmonary complications and postoperative mortality) all favored D1 dissection, (D2 vs. D1, 37% vs. 21%; RR 0.62, 95% CI: 0.5-0.76), while perioperative hemorrhage risks were equivalent. Subgroup analysis would suggest that D2 dissection, without spleen and pancreas resection, is better tolerated with a trend towards lower gastric cancer mortality (D2 vs. D1, 41% vs. 48%; RR 1.19, 95% CI: 0.98-1.44).
Notwithstanding these conclusions, the modest cure rate achievable for most locally advanced cancers despite complete surgical resections (R0), the desire to optimize surgery by adhering to sound oncological principles, the subgroup data that suggest superior survival when D2 dissection is used (without routine splenectomies and pancreatectomies) provide the justification to advocate for gastrectomy with D2 dissections, in expert hands, as the optimal surgical standard. Indeed, using a RAND/UCLA appropriateness study design, an expert panel considered D2 lymphadenectomy in all patients with tumors >T1N0. The panel also found the use of total gastrectomy for all patients and distal gastrectomies for patients with distal gastric cancers as appropriate (17).
Whether the factors leading to variations in surgical decisions were related to patient comorbidities, tumor extent or surgical expertise, different quality and extent of surgery is expected to have an impact on survival, treatment related morbidity and mortality and postoperative functional status. For patients with significant morbidities in the postoperative setting, many would not be suitable for additional adjunctive therapies even if there were indications to consider them. Judicial use of prognostic factors and clinical experience is the cornerstone for choosing the best approaches for individual patients.
What is the role of RT?
RT, a locoregional treatment, is likely to be most impactful if there is a significant risk of local regional recurrence despite optimal surgery. This may occur as a result of seeding of the tumor bed, challenges in achieving good resection margin clearance, or microscopic residual lymphatic involvement. The rationale for the optimal timing of RT, pre vs. postoperative, and the optimal way of combining systemic therapies with RT hinges on a complex relationship between the modalities, additive or synergistic, and the effect on anticipated toxicities and relative therapeutic ratio. These factors will be discussed in the following section, followed by a discussion of the existing evidence, and ongoing trials.
How effective is the state of the art surgery in securing local control?
Locoregional recurrence rates are often subject to detection and reporting biases. They are most likely to exist when locoregional recurrence pattern is not planned as an important outcome and where follow-up practices are not standardized. Consequently, some studies report on the site of first recurrence only, while others on recurrences at any time if they were followed. Geographic misses in relation to the extent of surgery, and the extent of RT, is labor intensive and generally not available to guide modifications on treatment delivery. Notwithstanding these biases, locoregional recurrence rates in the surgery alone arm are on the order of 20% (18) to 70% (19) depending on the quality and extent of the surgery. Even if we restrict our focus to trials with a high compliance for D2 dissections, locoregional recurrence remains a significant problem with a range of 32-42% (20). This pattern of locoregional recurrence would suggest a high potential that RT can have a major role in optimizing the management of patients with locally advanced gastric cancer (Table 1).
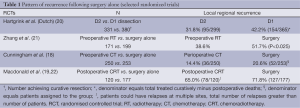
Full table
Pros and cons of pre vs. postoperative RT—general principles
The issue of whether RT is best employed in the preoperative or postoperative setting [with or without chemotherapy (CT)] has been the subject of debate in the management of many cancers such as rectum (23), sarcoma (24), and esophageal cancer (25) to name a few. Some general principles apply (Table 2).

Full table
The accuracy of clinical staging, typically based on diagnostic tests, plays an important role in identifying the appropriate patients for preoperative therapy, avoiding over treatment of early stage patients and the futile use of curative strategies in those who are harboring more advanced metastatic disease. For gastric cancer patients, the use of gastric protocols in the CT acquisition, incorporation of endoscopic ultrasound, laparoscopy and peritoneal washings are practices that are increasingly sophisticated to allow accurate preoperative staging.
The toxicity burden of multimodal therapies may differ based on the symptom profile and premorbid condition of the patient. Careful consideration of patients’ baseline condition and suitability for combined modality is necessary to avoid unacceptable treatment related morbidity and mortality. Borderline patients taken through preoperative therapy may delay or preclude the definitive surgery. Some patients with acute complications from the primary (e.g., uncontrolled bleeding, obstruction) demand immediate surgery even if preoperative therapy may have a role to play. Postoperative therapy typically needs to be given within a finite period following surgery (e.g., 10 weeks) beyond which the anticipated benefits are expected to diminish. In the original Macdonald trial 17% of patients stopped treatment because of toxicity, while major (≥ grade 3) toxicity occurred in 33% of patients.
The design of the RT target volume requiring treatment is generally smaller in the preoperative setting. The presence of the tumor typically displaces and minimizes the need to encompass normal structures (e.g., small bowel). In contrast, postoperative treatment typically requires inclusion of normal structures that would fill to original tumor site, and difficult to avoid when the tumor bed needs to be included. Surgery can open previously uninvolved planes that become potential routes of spread. Anastomosis and reconstructions may result in regions of interest located adjacent to sensitive structures (e.g., duodenal blind loop and its relationship to the L kidney, esophagogastric anastomosis), requiring expansion of treatment fields or suboptimal coverage of critical structures.
Finally, preoperative strategies generally require lower doses to achieve the same local control effect, with obvious benefits on the long term anticipated effect following treatment. This phenomenon is likely attributable to the increase in hypoxic tissues in the postoperative state.
What is the evidence?
In an attempt to clarify the role of RT for gastric cancer, for the purpose of this review, emphasis is placed on randomized trials that target the current definition (TNM 7th edition) of gastric cancer. Where GE or esophageal cancers represent >30% of the participants, the trials were excluded (unless subgroup data is available for gastric cancers). Similarly, systematic reviews, and meta-analyses that collate the evidence that emphasizes this body of primary studies are preferentially discussed. Clinicaltrial.gov was search for ongoing trials. Medline and Cochrane databases were searched. Guidelines Clearinghouse was searched for current evidence based guidelines (last searched Jun 2014).
A total of 16 randomized trials (11,21,26-39), four systematic reviews (40-43) addressing the role of RT in gastric cancer were identified with the most recent one published in 2014 (40). A single practice guideline (44) that is relevant to our question is listed under the National Guidelines Clearinghouse (45) and is included. A summary of the relevant references in the different study designs is included (Table 3).
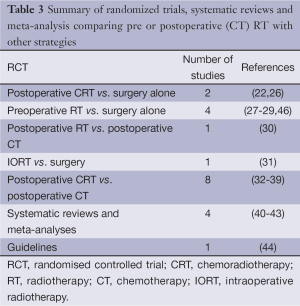
Full table
Preoperative RT vs. surgery alone (Tables 4,5)
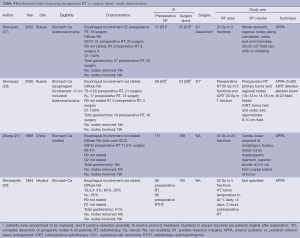
Full table
Full table
Preoperative RT is the subject of investigation in four randomized trials. The studies were performed in Russia, Ukraine and China and published between 1994 and 2002. The quality of reporting is generally poor with limited information on the quality of the surgery, adequacy of nodal dissection and extent of tumor involvement especially when contrasted against contemporary standards. With the exception of the study from China with a sample size of 370 patients, the studies were small (and likely underpowered). None of the studies provided a justification for the sample size design. The dose fractionation used was hypofractionated (2 Gy in 5 fractions) with the addition of intraoperative RT. In one (28), and the addition of hyperthermia in another study (29). The study from China employed a dose fractionation of 40 Gy in 20 fractions. The techniques used were all simple with anterior posterior vs. posterior anterior beam arrangement (APPA) techniques to upper abdominal fields that have generally been replaced by more sophisticated planning techniques.
Notwithstanding the significant risk of bias inherent within these trials, the study by Zhang et al. (21), the largest within this group, observed a survival benefit of approximately 7% (10 years OS: 20% preoperative RT vs. 13% surgery alone; P<0.05), using a modest dose of 40 Gy in 20 fractions.
A meta-analysis performed by Fiorica et al. (41) in 2007 provided summary statistics across the relevant trials showing a survival benefit with RT alone with a odds ratio (OR) 0.54, 95% CI: 0.43-0.68 (41). A more recent update by Pang et al. in 2014 using a different set of selection criteria arrived at a similar observation and conclusions (40).
While the primary preoperative RT studies were conducted with less sophisticated RT techniques and quality of surgery, the observation remains potentially compelling that modest doses of local regional RT delivered prospectively can complement surgery to provide a survival advantage. It is tantalizing to hypothesize that with optimal combination quality surgery and CT; more significant gains can be accomplished.
Postoperative CRT vs. surgery
The pivotal postoperative CRT vs. surgery trial (INT0116) was first reported by Macdonald et al. (11) resulting in the general adoption of postoperative CRT in addition to surgery as the standard treatment for gastric cancer in North American and Europe. Updated results were subsequently published (22) with a median follow up of more than 10 years, confirming the original observation of OS benefit of 9% with a hazard ratio (HR) 1.32 (95% CI: 1.1-1.6; P=0.0046). Relapse free survival (RFS) was 11% with a HR of 1.51 (95% CI: 1.25-1.83; P<0.001). The pattern of recurrence, with 24% fewer relapses occurring in patients in the CRT arm, confirmed the degree of benefit predicted through the original pattern of failure analysis by Gunderson et al. in 1982 (47). Subgroup analysis showed patients with diffuse histology (typically associated with poorer prognosis occurring in younger, female patients) appear to benefit less, while patients with more nodes (N4+ vs. others) derived greater benefit. The authors suggested extreme caution in their interpretation given the small numbers within some of the subgroups (22). Moertel et al. (48) also in this category is of historic interest only and is not discussed further.
Postoperative RT vs. postoperative CT
Hallissey et al. (30) reported on the second British stomach cancer trial comparing postoperative RT alone with postoperative CT. Patients were randomized to one of three arms, surgery alone, postoperative RT (45 Gy in 25 fractions, boost 5 Gy) vs. postoperative CT [mitomycin, doxorubicin and 5-fluorouracil (5FU)]. Proportion of patients with GE junction tumor was not stated. No survival advantage can be seen for 5 years OS (surgery vs. pRT vs. pCT: 20% vs. 12% vs. 19%).
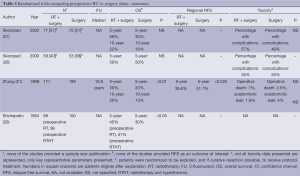
Postoperative CRT vs. postoperative CT (Tables 6,7)
Full table
Full table
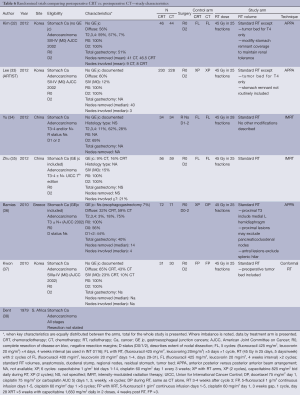
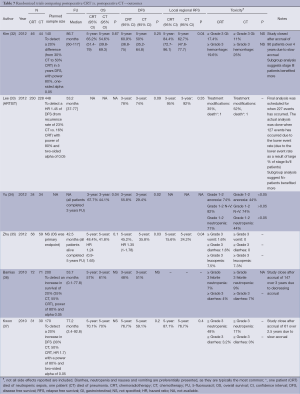
Six studies were designed to examine the incremental role of RT when added to postoperative CT and is the most frequently studied strategy in recent years, with four published in 2012 and two in 2010. In four of the studies, only patients who had a D2 dissection were included (32,33,35,37). Similarly, the RT used was most consistent with contemporary practice. All studies used a dose fractionation of 45 Gy in 25 fractions. All studies employed treatment targets consistent with standard practice (anastomosis, duodenal stump, local regional nodes, residual stomach, and tumor bed) with some modifications. Kim (32) and Lee (33) and Kwon (32,33,37) all excluded tumor bed treatments with the exception of T4 lesions. Coverage of the stomach remnant is more flexible permitting variations in favor of reducing dose to normal structures (e.g., kidneys). Two studies used intensity-modulated radiation therapy (IMRT) (34,35), one conformal RT (37) while three used older techniques (APPA) (32,33,36).
The different CT regimens used and the discussion around the optimal one is presented in the next section.
All but one study was underpowered. Three studies closed prematurely and lack the power to detect the difference they were looking for (32,36,37) and two (34,35) were small and almost certainly also underpowered. The ARTIST trial reported by Lee et al. (33) was the only study that successfully completed accrual and dominated this group of studies with 458 participants. It also suffered from sample size issues, with an unexpectedly high proportion of earlier stage tumors resulting in a lower event (recurrence) rate than anticipated.
There is some evidence to support improvements in local regional control (32,35) although the largest study (ARTIST) (33) did not find this benefit. While local regional control was extremely high (92% CT, 95% CRT) in the ARTIST trial, local RFS ranged from 63% CT to 84% CRT supporting the potential in improving outcomes by RT. No difference in survival, RFS and local regional relapse free was observed.
Choice of systemic regimen
5FU has been the mainstay chemotherapeutic agent when used concurrently with radiation, either in bolus form at the beginning and end of radiation (11,32,34,35), continuous infusional form (49), or in oral form as capecitabine (33,37). However, the CT before and after the radiation has been more varied. Other than 5FU (11,32,34,35), the following other CT regimens have been used: epirubicin, cisplatin and 5FU (ECF) (49), capecitabine and cisplatin (33), 5FU and cisplatin (37), as well as cisplatin and docetaxel (36).
Two additional important trials need to be considered when addressing the choice of systemic backbone when combined with RT. The MAGIC trial (18) established the survival benefit provided by ECF perioperative CT compared with surgery alone. A survival benefit was clearly established (5 years OS, 36% CT vs. 23% surgery alone; HR 0.75, 95% CI: 0.6-0.83; P=0.009), as well as an advantage in progression free survival (HR 0.66; 95% CI: 0.53-0.71; P<0.001). The CALBG trial (49) was the only phase III trial that compared the optimal CT when used in conjunction with postoperative radiation. The control arm used 5FU as in the Macdonald protocol, while the experimental arm used ECF CT before and after RT. Both arms used infusional 5FU during radiation (as opposed to bolus 5FU at the beginning and end of RT). Both groups had similar OS, and therefore the trial did not meet its primary endpoint. However, toxicity was reported to be less in the ECF arm, and the final publication is awaited.
At Princess Margaret, we still use 5FU as per the Macdonald protocol, as this has the best and longest standing evidence. However, others have switched to infusional 5FU during RT, as is often done in other gastrointestinal cancers such as rectal cancer, and some other centers have used ECF before and after radiation.
In the ongoing trials, TOPGEAR (50) is designed with perioperative ECF (6 cycles) vs. the same regimen replacing the 3rd cycle of ECF with RT with 5FU or capecitabine. CRITICS (51) employs a similar strategy using epirubicin, cisplatin and capecitabine (ECC) ×3 cycles, vs. the same regimen with RT and concomitant cisplatin and capecitabine. Zhou (52) and Xie (53) et al. use 2 cycles of capecitabine and oxaliplatin (CapOx), Kang et al. use cisplatin and capecitabine in one study (54), and S1 and oxaliplatin in ARTIST II (55). Biological agents are actively being investigated especially in North America (Table 8).
Full table
Summary
Taken together, these trials showed an interest in the use of preoperative RT (reported between 1994 and 2002), although perhaps given the quality of the evidence and the variable results, the findings were not translated into adoption of this strategy into clinical practice. The Macdonald study [2002] single handedly changed clinical practice to the adoption of postoperative CRT with a 9% survival benefit. Recent efforts (reported between 2010 and 2012), employing contemporary surgery, RT and “standard” CT, were focused on establishing the incremental benefit of adding RT to CT in the postoperative setting, found improved local control, but no survival benefit. A single small dated study [1994] would suggest postoperative RT alone to be ineffective.
Preoperative RT alone offered some tantalizing evidence that it can also improve survival but the power of inference is lower. The significant local regional rates that are expected from locally advanced disease despite improved surgical quality (including safe delivery of D2 dissections) are powerful reasons to motivate a strong support for current studies that are designed to establish the effectiveness of preoperative CRT when used together with optimized CT and surgery.
Technical considerations of RT
Choice of dose fractionation
The typical dose fractionation of 45 Gy in 25 fractions is employed quite uniformly across current practice and in ongoing clinical trials, given the relatively large target volume (driven by the distribution of local regional nodes predominantly), and the intimate relationship with critical normal structures and their normal tissue tolerances.
Choice of target volume
The choice of target volume is based on the principle to include all the local regional nodes at risk and the threat posed by direct microscopic extension.
Nodal regions encompassed would parallel what would be captured in an extended D2 dissection, where perigastric, celiac axis, pancreaticoduodenal, porta hepatis, are targeted. Paraaortic nodes are included where this corresponds to the cranial caudal extent of the overall target volume. Splenic hilar nodes are included in proximal tumors.
To account for the risk of recurrence arising through direct extension of the primary, a margin surrounding the primary (in the preoperative setting), or a margin around the perioperative tumor bed, residual stomach and excision margins on the tumor side, i.e., the anastomosis, and blind loop of the duodenum are used in the postoperative setting. The proximal hemi diaphragm is targeted for the same reasons in proximal tumors. In general terms, a clinical target volume (CTV) margin of 0.5-1 cm around the vasculature is used to capture the nodal groups. A margin of 0-0.5 cm around the primary for T1-2 lesions, and a margin of 0.5-1 cm for T3-4 primaries are typically used.
Certain modifications of these principles are generally permitted to reduce dose to normal structures under specific circumstances. For patients who have undergone a D2 dissection with adequate nodal sampling, omitting the preoperative tumor bed when the tumor is T3 or less, and omission of the entire residual stomach, are acceptable variations introduced in recent trials (32,33) with no adverse consequences reported.
At the conclusion of TOPGEAR, this study would have accrued 750 patients whereby half of the patients would have received preoperative CRT according to the method of target definition, with a thoughtful quality assurance program and is anticipated to provide high quality evidence on the appropriateness and effectiveness of the contouring guidelines used in this study.
Choice of treatment technique
When preoperative CRT was first introduced, the Macdonald trial described the use of APPA or three field techniques (19). This is quickly superseded by the adoption of conformal techniques, intensity modulated and volumetric arc techniques.
With more sophisticated treatment approaches, special considerations need to be made during planning and treatment delivery to ensure reproducible and accurate targeting. Dietary guidelines are an attempt to ensure minimal and consistent stomach volumes throughout the planning and treatment period. At our institution, a cup of coffee and a slice of toast (or its equivalent) only 2 hours prior to RT is routinely recommended. Daily image guidance incorporating cone beam computed tomography is necessary to provide verification of fields designed with more sophisticated planning techniques with sharper dose gradients (e.g., conformal, IMRT) to avoid normal structures. Renal perfusion scan can provide differential renal function and is useful for refining beam geometry and permissible dose to the kidneys. Four dimensional-computed tomography scans provide individualized assessment of respiratory organ motion assessment and planning target volume (PTV) margins (56).
A recent systematic review on comparison between standard and conformal three dimensional (3D) techniques supported superior normal tissue sparing with 3D CRT (57). More sophisticated techniques such as IMRT and tomotherapy, provide refinement in dosimetric advantages which could benefit particularly challenging cases although clinically significant differences at a population level is more difficult to demonstrate (58,59).
Ongoing phase III studies
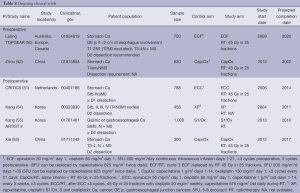
Globally, five randomized trials (50-53,55) are currently actively accruing, and one has completed accrual (54) and awaiting follow-up. Two studies examining the role of neoadjuvant RT when added to CT, and four studies addressed the role of RT in the adjuvant setting when added to CT.
Postoperative CT ± RT
Kang et al. (54) has completed accrual in 2011 on a study in Korea comparing capecitabine, cisplatin (XP), with or without RT having recruited 458 patients, results pending. A second study by the same group (55) aims to accrue 1,000 patients, comparing S1/oxaliplatin with or without RT, scheduled to complete accrual in 2016. Xie et al. (53) is conducting a study in China targeting 300 patients comparing capecitabine/oxaliplatin with or without RT, scheduled to completed in 2017.
CRITICS (Clinicaltrials.gov NCT00407186) (51) is designed to compare perioperative CT with postoperative CRT uses 45 Gy in 25 fractions (with cisplatin and capecitabine), together with high quality surgery, pathology and RT quality control. This study initiated accrual in 2006 and is scheduled to complete accrual of its sample size of 788 patients.
Preoperative CT ± RT
Zhou et al. is conducting a study in China comparing capcitabine/oxaliplatin in the preoperative setting in 620 patients, targeting completion of accrual in 2022 (52).
TOPGEAR (Clinicaltrial.gov NCT01924819) (50) is designed to deliver 45 Gy in 25 fractions, with 5FU in the preoperative setting during what would be the 3rd cycle of MAGIC CT. D2 dissection is strongly recommended. This study initiated accrual in 2009, and is scheduled to complete accrual of its sample size of 752 patients in 2020.
The design of this study is built upon three phase II studies providing promising safety data. Postoperative use of CRT using ECF was tested in a phase II study demonstrating tolerability (60) ECF ×1 cycle followed by CRT (45 Gy in 25 fractions with concurrent 5FU) was tested in the phase II setting through TROG 03.02. The definition of the RT target volumes and normal tissue dose limits and general planning approach provided evidence of initial safety and feasibility. In this study, compliance rate of 94% was achieved, and grade 3-4 gastrointestinal (GI) toxicity was 28% and neutropenia 65%, febrile neutropenia 5.6% (60). Ajani et al. (61) reported on the first of two multi-institutional phase II neoadjuvant study (n=34) using 5FU/folinic acid (FA)/cis-diamminedichloroplatinum (CDDP) followed by CRT (45 Gy in 24 fractions with concurrent continuous intravenous infusion 5FU). The R0 resection rate was 70% and the pathological complete response (pCR) rate was 30% while median survival was 34 months. The second phase II study (62) (RTOG 99-04) (n=49) used 5FU/FA/CDDP ×2 cycles preoperative, followed by CRT (45 Gy with concurrent continuous intravenous infusion 5FU/paclitaxel). The R0 resection rate was 77%, pCR 26%. Both studies reported an acceptable toxicity profile.
Conclusions
Differences in patterns of practice have resulted in different strategies to enhance the outcome of surgery between the East and the West. TNM staging system version 7 published in 2010 represent a consensus between these two worlds and would likely lay the foundation for advances that would capitalize on these variations. The philosophy that quality is important, especially in technical based modalities such as RT and surgery is critical, if optimal effect of combined modality is to be defined.
The technical ability to deliver RT to large complex volumes while minimizing exposure to normal structures has matured. Postoperative CRT improves the cure rate by approximately 9%, attributable to the effect of RT on securing local control when the majority of patients are managed by D0-1 dissections. Ongoing trials are expected to provide the answer to the question, what is the role of incorporating RT and CT to optimal surgery in both the preoperative or postoperative setting over the next 5-10 years. Based on sound principles, there is particular optimism that preoperative CRT may have a critical role to play. Assuming safety and effectiveness is confirmed in the neoadjuvant setting, future trials would need to be initiated to clarify the role between pre and postoperative RT.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Cancer IAfRo. Globocan 2012 [cited 2014 Jul 4]; Available online: http://globocan.iarc.fr/Default.aspx
- Biondi A, Hyung WJ. Seventh edition of TNM classification for gastric cancer. J Clin Oncol. 2011;29:4338-9; author reply 4340-2.
- Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol 2010;17:3077-9.
- Lee SM, Kim KM, Ro JY. Gastric Carcinoma: Morphologic Classifications and Molecular Changes. In: Lazăr D. eds. Gastric Carcinoma-New Insights into Current Management. InTech: Available online: http://www.intechopen.com/books/gastric-carcinoma-new-insights-into-current-management/gastric-carcinoma-morphologic-classifications-and-molecular-changes; 2013.
- Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol 2013;107:230-6. [PubMed]
- Bittoni A, Scartozzi M, Giampieri R, et al. Clinical evidence for three distinct gastric cancer subtypes: time for a new approach. PLoS One 2013;8:e78544. [PubMed]
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [PubMed]
- Bang YJ, Yalcin S, Roth A, et al. Registry of gastric cancer treatment evaluation (REGATE): I baseline disease characteristics. Asia Pac J Clin Oncol 2014;10:38-52. [PubMed]
- Murakami T. Pathomorphological diagnosis. Definition and gross classification of early gastric cancer. Gann Monogr Cancer Res 1971;11:53-5.
- DE Sol A, Trastulli S, Grassi V, et al. Requirement for a standardised definition of advanced gastric cancer. Oncol Lett 2014;7:164-70. [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. [PubMed]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14:101-12. [PubMed]
- Son T, Hyung WJ, Kim JW, et al. Anatomic extent of metastatic lymph nodes: still important for gastric cancer prognosis. Ann Surg Oncol 2014;21:899-907. [PubMed]
- Eom BW, Joo J, Kim YW, et al. Improved survival after adding dissection of the superior mesenteric vein lymph node (14v) to standard D2 gastrectomy for advanced distal gastric cancer. Surgery 2014;155:408-16. [PubMed]
- Seevaratnam R, Bocicariu A, Cardoso R, et al. How many lymph nodes should be assessed in patients with gastric cancer? A systematic review. Gastric Cancer 2012;15 Suppl 1:S70-88. [PubMed]
- Jiang L, Yang KH, Chen Y, et al. Systematic review and meta-analysis of the effectiveness and safety of extended lymphadenectomy in patients with resectable gastric cancer. Br J Surg 2014;101:595-604. [PubMed]
- Brar S, Law C, McLeod R, et al. Defining surgical quality in gastric cancer: a RAND/UCLA appropriateness study. J Am Coll Surg 2013;217:347-57.e1.
- Cunningham D, Jost LM, Purkalne G, et al. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of gastric cancer. Ann Oncol 2005;16 Suppl 1:i22-3. [PubMed]
- Macdonald JS. Role of post-operative chemoradiation in resected gastric cancer. J Surg Oncol 2005;90:166-70. [PubMed]
- Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 2004;22:2069-77. [PubMed]
- Zhang ZX, Gu XZ, Yin WB, et al. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of adenocarcinoma of gastric cardia (AGC)--report on 370 patients. Int J Radiat Oncol Biol Phys 1998;42:929-34. [PubMed]
- Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 2012;30:2327-33. [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [PubMed]
- O’Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 2002;359:2235-41. [PubMed]
- Malthaner R, Wong RK, Spithoff K, et al. Preoperative or postoperative therapy for resectable oesophageal cancer: an updated practice guideline. Clin Oncol (R Coll Radiol) 2010;22:250-6. [PubMed]
- Moertel CG, Childs DS, O'Fallon JR, et al. Combined 5-fluorouracil and radiation therapy as a surgical adjuvant for poor prognosis gastric carcinoma. J Clin Oncol 1984;2:1249-54. [PubMed]
- Skoropad V, Berdov B, Zagrebin V. Concentrated preoperative radiotherapy for resectable gastric cancer: 20-years follow-up of a randomized trial. J Surg Oncol 2002;80:72-8. [PubMed]
- Skoropad VY, Berdov BA, Mardynski YS, et al. A prospective, randomized trial of pre-operative and intraoperative radiotherapy versus surgery alone in resectable gastric cancer. Eur J Surg Oncol 2000;26:773-9. [PubMed]
- Shchepotin IB, Evans SR, Chorny V, et al. Intensive preoperative radiotherapy with local hyperthermia for the treatment of gastric carcinoma. Surg Oncol 1994;3:37-44. [PubMed]
- Hallissey MT, Dunn JA, Ward LC, et al. The second British Stomach Cancer Group trial of adjuvant radiotherapy or chemotherapy in resectable gastric cancer: five-year follow-up. Lancet 1994;343:1309-12. [PubMed]
- Krämling HJ, Wilkowski R, Dühmke E, et al. Adjuvant intraoperative radiotherapy of stomach carcinoma. Langenbecks Arch Chir Suppl Kongressbd 1996;113:211-3. [PubMed]
- Kim TH, Park SR, Ryu KW, et al. Phase 3 trial of postoperative chemotherapy alone versus chemoradiation therapy in stage III-IV gastric cancer treated with R0 gastrectomy and D2 lymph node dissection. Int J Radiat Oncol Biol Phys 2012;84:e585-92. [PubMed]
- Lee J. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 2012;30:268-73. [PubMed]
- Yu C, Yu R, Zhu W, et al. Intensity-modulated radiotherapy combined with chemotherapy for the treatment of gastric cancer patients after standard D1/D2 surgery. J Cancer Res Clin Oncol 2012;138:255-9. [PubMed]
- Zhu WG, Xua DF, Pu J, et al. A randomized, controlled, multicenter study comparing intensity-modulated radiotherapy plus concurrent chemotherapy with chemotherapy alone in gastric cancer patients with D2 resection. Radiother Oncol 2012;104:361-6. [PubMed]
- Bamias A, Karina M, Papakostas P, et al. A randomized phase III study of adjuvant platinum/docetaxel chemotherapy with or without radiation therapy in patients with gastric cancer. Cancer Chemother Pharmacol 2010;65:1009-21. [PubMed]
- Kwon HC, Kim MC, Kim KH, et al. Adjuvant chemoradiation versus chemotherapy in completely resected advanced gastric cancer with D2 nodal dissection. Asia Pac J Clin Oncol 2010;6:278-85. [PubMed]
- Dent DM, Werner ID, Novis B, et al. Prospective randomized trial of combined oncological therapy for gastric carcinoma. Cancer 1979;44:385-91. [PubMed]
- A comparison of combination chemotherapy and combined modality therapy for locally advanced gastric carcinoma. Gastrointestinal Tumor Study Group. Cancer 1982;49:1771-7. [PubMed]
- Pang X, Wei W, Leng W, et al. Radiotherapy for gastric cancer: a systematic review and meta-analysis. Tumour Biol 2014;35:387-96. [PubMed]
- Fiorica F, Cartei F, Enea M, et al. The impact of radiotherapy on survival in resectable gastric carcinoma: a meta-analysis of literature data. Cancer Treat Rev 2007;33:729-40. [PubMed]
- Valentini V, Cellini F. Radiotherapy in gastric cancer: a systematic review of literature and new perspectives. Expert Rev Anticancer Ther 2007;7:1379-93. [PubMed]
- Ohri N, Garg MK, Aparo S, et al. Who benefits from adjuvant radiation therapy for gastric cancer? A meta-analysis. Int J Radiat Oncol Biol Phys 2013;86:330-5. [PubMed]
- Knight G, Earle CC, Cosby R, et al. Neoadjuvant or adjuvant therapy for resectable gastric cancer: a systematic review and practice guideline for North America. Gastric Cancer 2013;16:28-40. [PubMed]
- Agency for Healthcare Research and Quality. Quality AfHRa. National Guideline Clearinghouse. [cited 2014 Jul 4 ]; Available online: http://www.guideline.gov/index.aspx
- Zhang CD, Chen SC, Feng ZF, et al. Laparoscopic versus open gastrectomy for early gastric cancer in Asia: a meta-analysis. Surg Laparosc Endosc Percutan Tech 2013;23:365-77. [PubMed]
- Gunderson LL, Sosin H. Adenocarcinoma of the stomach: areas of failure in a re-operation series (second or symptomatic look) clinicopathologic correlation and implications for adjuvant therapy. Int J Radiat Oncol Biol Phys 1982;8:1-11. [PubMed]
- Moertel CG, Childs DS Jr, Reitemeier RJ, et al. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet 1969;2:865-7. [PubMed]
- Fuchs CS, Tepper JE, Niedzwiecki D, et al. Postoperative adjuvant chemoradiation for gastric or gastroesophageal junction (GEJ) adenocarcinoma using epirubicin, cisplatin, and infusional (CI) 5-FU (ECF) before and after CI 5-FU and radiotherapy (CRT) compared with bolus 5-FU/LV before and after CRT: Intergroup trial CALGB 80101. J Clin Oncol 2011;29:abstr 4003.
- Australasian Gastro-Intestinal Trials Group. Trial of preoperative therapy for gastric and esophagogastric junction adenocarcinoma (TOPGEAR). [cited 2014 Jul 1]; Available online: http://clinicaltrials.gov/show/NCT01924819
- Group DCC. Randomized Phase III Trial of Adjuvant chemotherapy or chemoradiotherapy in resectable gastric cancer (CRITICS). [cited 2014 Jul 4]; Available online: http://clinicaltrials.gov/ct2/show/NCT00407186
- Zhou Z. Pre-operative chemoradiotherapy or chemotherapy following surgery and adjuvent chemotherapy in patients with gastric cancer. [cited 2014 Jul 4]; Available online: http://clinicaltrials.gov/ct2/show/NCT01815853
- Xie C. Trial of adjuvant XELOX chemotherapy and concurrent capecitabine and radiotherapy for resected gastric carcinoma. [cited 2014 Jul 4]; Available online: http://clinicaltrials.gov/ct2/show/NCT01711242
- Kang WK. Phase III randomized trial of adjuvant XP chemotherapy and XP/RT for resected gastric adenocarcinoma. [cited 2014 Jul 4]; Available online: http://clinicaltrials.gov/ct2/show/NCT00323830
- Kang WK. Phase III randomized trial of adjuvant chemotherapy with S-1 vs. S-1/oxaliplatin ± radiotherapy for completely resected gastric adenocarcinoma: the ARTIST II trial (ARTIST-II). [cited 2014 Jul 4]; Available online: http://clinicaltrials.gov/ct2/show/NCT01761461
- Wysocka B, Kassam Z, Lockwood G, et al. Interfraction and respiratory organ motion during conformal radiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys 2010;77:53-9. [PubMed]
- Morganti AG, Di Castelnuovo A, Massaccesi M, et al. Planning comparison between standard and conformal 3D techniques in post-operative radiotherapy of gastric cancer: a systematic review. Br J Radiol 2013;86:20130274. [PubMed]
- Dahele M, Skinner M, Schultz B, et al. Adjuvant radiotherapy for gastric cancer: A dosimetric comparison of 3-dimensional conformal radiotherapy, tomotherapy and conventional intensity modulated radiotherapy treatment plans. Med Dosim 2010;35:115-21. [PubMed]
- Taremi M, Ringash J, Dawson LA. Upper abdominal malignancies: intensity-modulated radiation therapy. Front Radiat Ther Oncol 2007;40:272-88. [PubMed]
- Leong T, Joon DL, Willis D, et al. Adjuvant chemoradiation for gastric cancer using epirubicin, cisplatin, and 5-fluorouracil before and after three-dimensional conformal radiotherapy with concurrent infusional 5-fluorouracil: a multicenter study of the Trans-Tasman Radiation Oncology Group. Int J Radiat Oncol Biol Phys 2011;79:690-5. [PubMed]
- Ajani JA, Mansfield PF, Janjan N, et al. Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol 2004;22:2774-80. [PubMed]
- Ajani JA, Winter K, Okawara GS, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol 2006;24:3953-8. [PubMed]


