Robotic assisted Ivor Lewis esophagectomy in the elderly patient
Introduction
Esophageal cancer continues to increase in incidence worldwide (1-4). In the United States in 2013 there were 17,990 new cases of esophageal cancer and 15,210 deaths (4). The average age at the time of diagnosis continues to rise, and men and women are both presenting at an advanced age at the time of diagnosis, with a peak incidence between 75 and 79 years of age (1). The long-term survival for patients with locally advanced esophageal cancer remains poor despite improvements in multi-modality care over the last several decades. The current approach to locally advanced esophageal cancer includes neoadjuvant chemoradiation followed by surgical resection (5). Traditionally, older age has been associated with a presumed frailty and there is a concern that the elderly may not be able to tolerate the complex treatment regimen now recommended for esophageal cancer.
Minimally invasive esophagectomy (MIE), including robotic assisted techniques, offer several potential advantages over traditional open esophagectomy. MIE has been found to result in faster recovery time, shorter hospitalization, and diminished post-operative pain. Biere et al. demonstrated favorable results for MIE in their open label controlled trial in which patients were randomized to either open esophagectomy or MIE. They reported patients undergoing MIE were less likely to have pulmonary infections and had shorter hospital stays compared to patients undergoing open esophagectomy (6). Additionally, retrospective reviews have demonstrated MIE does not compromise oncologic principles and is safe compared to traditional open esophagectomy for esophageal cancer (7-11). Robotic assisted Ivor Lewis esophagectomy (RAIL) is a new technique that allows the surgeon a broader three-dimensional view of the operative field with the added benefit of improved instrument articulation and motion over standard thoracoscopy.
We have previously described the development and implementation of RAIL, however, the specific use of this technique in the elderly has not been extensively reviewed (12). We sought to evaluate outcomes after RAIL across all age groups to determine if this approach is safe in the elderly.
Methods
A retrospective review of all consecutive patients undergoing RAIL from 2009 to 2013 was conducted after obtaining study approval from our Institutional Review Board. All patients regardless of age, race, tumor stage or location, or receipt of neoadjuvant therapy were included in the cohort. Patients were required to have a tissue diagnosis of cancer, but were not excluded based upon histologic variant. Basic demographics, tumor characteristics, operative details, and post-operative outcomes were recorded.
The patients were analyzed as an entire cohort and then divided into three separate cohorts based upon age. Cohorts were defined as follows: cohort 1, ≤49 years old; cohort 2, 50 to 69 years old; and cohort 3, ≥70 years old. A separate analysis was performed evaluating outcomes of the elderly, defined as patients ≥70 years of age, compared to those patients ≤69 years of age.
Endpoints and statistical analysis
The primary endpoints were median operating room (OR) time, estimated blood loss (EBL), intensive care unit (ICU) days following surgery, and length of hospitalization (LOH). Secondary end-points included peri-operative adverse events (AE) less than 30 days following surgery; including pneumonia, cardiac arrhythmia, deep vein thrombosis (DVT)/pulmonary embolism (PE), wound infection, leak, and death.
Statistical analysis was performed using SPSS® version 21.0 (IBM®, Chicago, IL, USA). Continuous variables were compared using the Kruskal Wallis or the ANOVA tests as appropriate. Pearson’s Chi-square test was used to compare categorical variables. All statistical tests were two-sided and an α (type I) error <0.05 was considered statistically significant.
Results
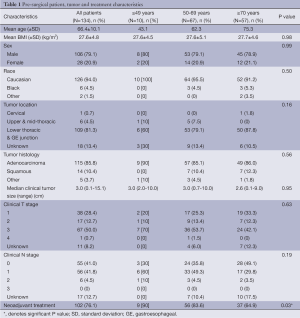
We identified 134 patients (106 men, 28 women) who underwent RAIL during the study period. The average patient age was 66±10 years (Table 1). Adenocarcinoma was the predominant histology and was diagnosed in 115 (86%) patients. Only 14 (10%) patients had squamous cell histology and 5 (4%) patients had other histology. Neoadjuvant therapy was administered to 102 (76%) patients. All patients underwent a complete resection (R0) and the median tumor size was 3.0 (range, 0.1-15.1) cm. The median OR time was 407 (range, 239-694) minutes with a median EBL of 150 (range, 25-600) mL. There were 5 (4%) leaks and 2 (1.5%) deaths in the entire cohort.
Full table
The patients were divided into three cohorts by age for comparison (Table 1). Ten patients were ≤49 years old (8 men, 2 women), 67 patients were 50 to 69 years old (53 men, 14 women) and 57 patients were ≥70 years of age (45 men, 12 women). The only statistically significant difference among the cohorts at baseline was the receipt of neoadjuvant therapy. Only 65% of patients ≥70 years old received neoadjuvant therapy compared to 90% of patients ≤49 years old and 84% of patients 50 to 69 years of age (P=0.03).
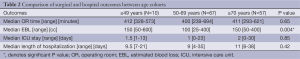
There was no significant differences between the three cohorts with respect to median or time, ICU days, or LOH (P=0.65, P=0.85, P=0.42, respectively, Table 2). There was, however, a significant difference in median EBL between the three age groups; patients aged 50 to 69 had the lowest amount of blood loss [100 (range, 25-400) mL] while patients ≤49 and ≥70 had a median EBL of 150 mL (range, 50-600 and 50-400 mL, respectively; P=0.004). Re-admission rates were low at 5.2% and did not vary amongst age groups. There were 0 (0%) in the ≤49, 4 (5.6%) in the 50-69, and 3 (5.7%) in the ≥70 age group P=0.52.
Full table
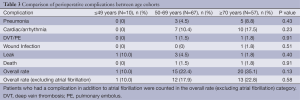
The rate of overall complication after surgery was not significantly different among the three cohorts (Table 3). Patients ≥70 years old had a higher absolute rate of overall complications (35%), although the difference in overall complication rate was not significant (P=0.13). Cardiac arrhythmias were the most frequent complication and were seen in 17 (12.7%) patients. Additionally, there was no significant difference in rate of pneumonia (P=0.43), wound infection (P=0.51), DVT or PE (P=0.91), leak (P=0.40), or death (P=0.91) among the three cohorts. Excluding cardiac arrhythmia, the overall rate of complications remained low and there was still no statistically significant difference between the three cohorts (≤49 years old 10%, 50 to 69 years old 18%, ≥70 years old 23%; P=0.58).
Full table
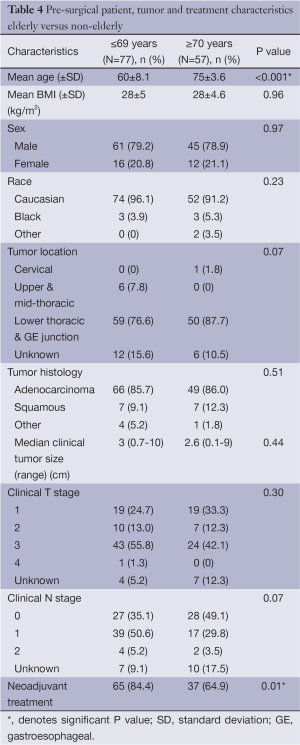
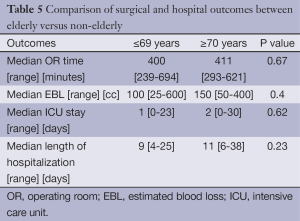
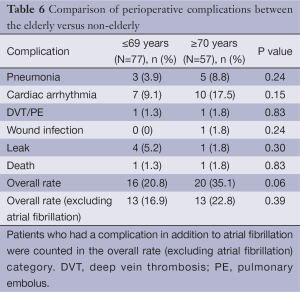
A separate analysis was done to compare the elderly (≥70 years old) to the non-elderly (≤69 years old). The only difference in baseline demographics between the two cohorts was once again receipt of neoadjuvant therapy (P=0.01) (Table 4). Median EBL was higher in the elderly cohort, but not statistically significant [100 (range, 25-600) vs. 150 (range, 50-400) mL; P<0.4]. There was also a trend toward longer LOH in the elderly [9 (range, 4-25) vs. 11 (range, 6-38) days; P=0.23 (Table 5)]. AE and mortality were not significantly different, although there was a trend towards increased AE (20.8% vs. 35.1%, P=0.06) in the cohort of patients ≥70 years of age, again with cardiac arrhythmia being the most common. There was a higher rate of cardiac arrhythmias in the patients who were ≥70 years old; 7 (9.1%) in the ≤ 69 group and 10 (17.5%) in the ≥70 cohort (P=0.15). The overall AE rate excluding cardiac arrhythmias was 13 (16.9%) in the ≤69 cohort vs. 13 (22.8%) in the ≥70 cohort P=0.39 (Table 6). Additionally, patients that developed a cardiac arrhythmia had a median length of hospitalization of 1.5 days longer than those who did not, 9 (range, 4-38) and 10.5 (range, 7-28) days respectively (P=0.07).
Full table
Full table
Full table
Discussion
We report our series of 134 RAIL cases comparing outcomes by increasing age. While the AE rates were higher amongst the ≥70 population, this was predominated by cardiac arrhythmias and was not statistically significant. When accounting for these arrhythmias, overall AE rates were no different between cohorts. Additionally, there were no significant differences in operative outcomes and LOH between the elderly vs. younger cohorts.
Surgical resection is an integral part of the treatment algorithm for early stage and locally advanced esophageal cancer. Unfortunately, the morbidity associated with esophagectomy can be high and is estimated in the literature to be between 25% and 50% (2,13,14). Pulmonary and cardiovascular complications such as atelectasis, pneumonia, and atrial fibrillation, in addition to wound infection, anastomotic leak, and chylothorax are among the most commonly seen post-operative complications and may increase the risk of mortality (13,15). As life expectancy increases, the average age at time of diagnosis is expected to continue to increase. This trend may have a significant impact on the treatment algorithm for elderly patients if age alone is determined to be an operative risk factor. Given that treatment regimens now call for multi-modality approaches including chemoradiation prior to surgery, the age of a patient has been called into question further as a potential risk factor for poor outcomes after treatment for esophageal cancer. The data to support this theory, however, is controversial.
Age has been demonstrated in several studies to correlate with higher rates of morbidity and mortality as well as worse survival (16-20). In their study of 474 patients undergoing esophagectomy between 2002 and 2011, Tapias et al. demonstrated an increased risk of morbidity and mortality in the elderly. The overall major complication rate was highest in the cohort over the age of 80 at 62.5% compared 47.6% for those 70 to 79 years of age, and 37.2% for patients less than 70 years old (P=0.016). Mortality was also significantly different, 0.6% for patients less than 70, 3.2% for those 70 to 79 years old, and 6.3% for the patients over age 80 (P=0.032). The majority of these cases were performed using an open Ivor Lewis technique (45.7%) and only 8% were MIE (19). Similarly, in an analysis of the Society of Thoracic Surgeons General Thoracic Database by Wright et al. in which 2,315 esophagectomy cases were reviewed, the authors found that age, cardiovascular disease, diabetes, and smoking were independent risk factors on multivariate analysis for increase morbidity and mortality (16).
Several other studies, however, have found that when adjusted for comorbid conditions, age itself is not a predictor of post-operative morbidity (2,21-26). In a review of 685 patients undergoing esophagectomy between 1994 and 2012 at a single institution cancer center, McLoughlin et al. found that the only significant predictor of overall survival and disease free survival on multivariate analysis was neoadjuvant therapy. Age was not found to be a significant predictor of adverse outcomes (P=0.66) (2). Pultrum et al. also concluded in their analysis of 234 patients that comorbid conditions, not age, were predictors of complications, and they found no difference in rates of in-hospital mortality or overall number of complications. Additionally, the presence of a comorbid condition, not age, was an independent prognostic factor for survival (21).
Age and comorbid status may have an impact on outcomes after open esophagectomy, however, MIE may provide a reduction in the risk of complications to this patient population. Outcomes after MIE have been well-studied and found to be equivalent in safety and efficacy when compared with open procedures while providing shorter hospitalization, reduction in pain and need for narcotic medication, and a faster return to normal activity. In an early analysis of fifty patients undergoing RAIL at our institution, we found that lymph node yield (20.6±9.3) and percentage of microscopically negative margins (100%) indicated equivalence of robotic to open approach (1). In their 3-year results of robotic-assisted transhiatal esophagectomy, Dunn et al., achieved a similar lymph node yield [20 (range, 3-38)] and a 94.7% R0 resection rate (27). A study by Sihag et al. evaluated perioperative outcomes in 38 patients undergoing Ivor-Lewis MIE (combination of laparoscopy and thoracoscopy) compared to 76 patients undergoing open Ivor Lewis esophagectomy. They found no difference in adequacy of oncologic outcomes: median number of lymph nodes, resection margins, and 60-day mortality. The MIE group, however, had a significantly reduced risk of developing pulmonary complications and were also found to have reduced length of ICU and hospital stay (15).
The robotic approach does require technical expertise by the operating surgeon and an OR team familiar with the intricacies of using the robot such as set-up, docking, and instrument exchange. Efficacy and feasibility of robotic surgery for complex esophageal surgery has been evaluated and found to offer enhanced three-dimensional visualization and advanced articulation with wrist-like motion. The potential draw-back to adoption of this technique is the steep learning curve required to achieve proficiency (27-30). In our experience, there was a significant reduction in operative time after completing twenty cases (514 vs. 397 minutes, P<0.005). During our initial evaluation of outcomes after our first 52 cases, we reported one case of anastomotic leak, no deaths and the overall complication rate was low at (26.9%). However, once the learning curve was reached (after 29 cases) the overall morbidity decreased, [n=10 (34%) vs. 4 (19%); P=0.07]. Additionally, there were no conversions to open thoracotomy and all patients in the series received an R0 resection (29).
Age alone has not been definitively proven to contribute to worse outcomes for open esophagectomy, and MIE has demonstrated reduction in post-operative pulmonary complications and shorter hospitalization, however, the impact of age on MIE, specifically RAIL, has not been thoroughly evaluated. The purpose of this study was to demonstrate that RAIL is a safe and reasonable operative approach in elderly patients with esophageal cancer. We acknowledge the limitations of this study that include the retrospective nature of the review. This cohort includes all consecutive patients undergoing RAIL at a single institution where all procedures were performed by a single surgeon thereby minimizing selection bias or variation in operative technique or learning curve as a factor in analyzing outcome data.
Conclusions
In our series of 134 patients, we were able to demonstrate that RAIL is a safe surgical technique for use in elderly patients. This represents the largest series to date with the RAIL technique and we demonstrated that elderly patients undergoing RAIL do not experience longer operative times, length of time in an ICU or the hospital overall, nor have they been shown to suffer increased risk of complication or death. When separating the study groups into those greater than 70 and those less than 70 years old, there were trends toward significant differences in LOH and AE although this was related to the increasing incidence of cardiac arrhythmias in patients who are older than 70. Close monitoring and vigilant post-operative care are required to ensure safe outcomes after esophagectomy for all patients regardless of age.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Yamamoto M, Weber JM, Karl RC, et al. Minimally invasive surgery for esophageal cancer: review of the literature and institutional experience. Cancer Control 2013;20:130-7. [PubMed]
- McLoughlin JM, Lewis JM, Meredith KL. The impact of age on morbidity and mortality following esophagectomy for esophageal cancer. Cancer Control 2013;20:144-50. [PubMed]
- Hur C, Miller M, Kong CY, et al. Trends in esophageal adenocarcinoma incidence and mortality. Cancer 2013;119:1149-58. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Beasley GM, Speicher P, Sharma K, et al. Efficacy of repeat sentinel lymph node biopsy in patients who develop recurrent melanoma. J Am Coll Surg 2014;218:686-92. [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [PubMed]
- Santillan AA, Farma JM, Meredith KL, et al. Minimally invasive surgery for esophageal cancer. J Natl Compr Canc Netw 2008;6:879-84. [PubMed]
- Verhage RJ, Hazebroek EJ, Boone J, et al. Minimally invasive surgery compared to open procedures in esophagectomy for cancer: a systematic review of the literature. Minerva Chir 2009;64:135-46. [PubMed]
- Safranek PM, Cubitt J, Booth MI, et al. Review of open and minimal access approaches to oesophagectomy for cancer. Br J Surg 2010;97:1845-53. [PubMed]
- Nagpal K, Ahmed K, Vats A, et al. Is minimally invasive surgery beneficial in the management of esophageal cancer? A meta-analysis. Surg Endosc 2010;24:1621-9. [PubMed]
- de la Fuente SG, Weber J, Hoffe SE, et al. Initial experience from a large referral center with robotic-assisted Ivor Lewis esophagogastrectomy for oncologic purposes. Surg Endosc 2013;27:3339-47. [PubMed]
- Karl RC, Schreiber R, Boulware D, et al. Factors affecting morbidity, mortality, and survival in patients undergoing Ivor Lewis esophagogastrectomy. Ann Surg 2000;231:635-43. [PubMed]
- Berger AC, Bloomenthal A, Weksler B, et al. Oncologic efficacy is not compromised, and may be improved with minimally invasive esophagectomy. J Am Coll Surg 2011;212:560-6; discussion 566-8. [PubMed]
- Sihag S, Wright CD, Wain JC, et al. Comparison of perioperative outcomes following open versus minimally invasive Ivor Lewis oesophagectomy at a single, high-volume centre. Eur J Cardiothorac Surg 2012;42:430-7. [PubMed]
- Wright CD, Kucharczuk JC, O’Brien SM, et al. Predictors of major morbidity and mortality after esophagectomy for esophageal cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk adjustment model. J Thorac Cardiovasc Surg 2009;137:587-95; discussion 596. [PubMed]
- Cijs TM, Verhoef C, Steyerberg EW, et al. Outcome of esophagectomy for cancer in elderly patients. Ann Thorac Surg 2010;90:900-7. [PubMed]
- Moskovitz AH, Rizk NP, Venkatraman E, et al. Mortality increases for octogenarians undergoing esophagogastrectomy for esophageal cancer. Ann Thorac Surg 2006;82:2031-6. [PubMed]
- Tapias LF, Muniappan A, Wright CD, et al. Short and long-term outcomes after esophagectomy for cancer in elderly patients. Ann Thorac Surg 2013;95:1741-8. [PubMed]
- Poon RT, Law SY, Chu KM, et al. Esophagectomy for carcinoma of the esophagus in the elderly: results of current surgical management. Ann Surg 1998;227:357-64. [PubMed]
- Pultrum BB, Bosch DJ, Nijsten MW, et al. Extended esophagectomy in elderly patients with esophageal cancer: minor effect of age alone in determining the postoperative course and survival. Ann Surg Oncol 2010;17:1572-80. [PubMed]
- Ruol A, Portale G, Zaninotto G, et al. Results of esophagectomy for esophageal cancer in elderly patients: age has little influence on outcome and survival. J Thorac Cardiovasc Surg 2007;133:1186-92. [PubMed]
- Fang W, Igaki H, Tachimori Y, et al. Three-field lymph node dissection for esophageal cancer in elderly patients over 70 years of age. Ann Thorac Surg 2001;72:867-71. [PubMed]
- Zehetner J, Lipham JC, Ayazi S, et al. Esophagectomy for cancer in octogenarians. Dis Esophagus 2010;23:666-9. [PubMed]
- Kiernan PD, Khandhar SJ, Fortes DL, et al. Thoracic surgery in octogenarians: CVTSA/Inova Fairfax hospital experience, 1990 to 2009. Am Surg 2011;77:675-80. [PubMed]
- Morita M, Egashira A, Yoshida R, et al. Esophagectomy in patients 80 years of age and older with carcinoma of the thoracic esophagus. J Gastroenterol 2008;43:345-51. [PubMed]
- Dunn DH, Johnson EM, Morphew JA, et al. Robot-assisted transhiatal esophagectomy: a 3-year single-center experience. Dis Esophagus 2013;26:159-66. [PubMed]
- Boone J, Schipper ME, Moojen WA, et al. Robot-assisted thoracoscopic oesophagectomy for cancer. Br J Surg 2009;96:878-86. [PubMed]
- Hernandez JM, Dimou F, Weber J, et al. Defining the learning curve for robotic-assisted esophagogastrectomy. J Gastrointest Surg 2013;17:1346-51. [PubMed]
- Weksler B, Sharma P, Moudgill N, et al. Robot-assisted minimally invasive esophagectomy is equivalent to thoracoscopic minimally invasive esophagectomy. Dis Esophagus 2012;25:403-9. [PubMed]


