
BRAF-V600E and microsatellite instability prediction through CA-19-9/CEA ratio in patients with colorectal cancer
Introduction
Colorectal cancer (CRC) is a heterogeneous disease involving various genomic alterations including but not limited to APC, TP53, KRAS, PIK3CA, SMAD4, and BRAF genes. Among them, CRC with BRAF V600E mutation is associated with the worst survival and poor prognosis (1). Typically, upfront triplet chemotherapy with an anti-VEGF agent is the preferred approach. Recently targeted therapy approaches utilizing anti-EGFR alongside BRAF-inhibitors are now included in the guidelines for after failure of first line therapy. On the other hand, CRC patients with mismatch repair deficiency or microsatellite instability-high (dMMR/MSI-High) have a high tumor mutation burden and respond dramatically to immunotherapy, and not as well to chemotherapy (2). So, early identification of these two subsets of patients has both prognostic and predictive value. Tissue biopsy with molecular profiling and mutational analysis is the standard technique used for grouping and analyzing the tumors. Liquid biopsies (circulating tumor DNA testing—ctDNA testing) are also increasingly being used.
We wanted to highlight an observation of utilizing 2 simple, rapid and universally available lab tests, i.e., carbohydrate cancer antigen 19-9 (CA 19-9) and carcinoembryonic antigen (CEA) tumor markers, the ratio (CA-19-9/CEA) of which can distinctly identify these subsets of patients.
Methods
Patients with metastatic CRC at Mayo Clinic from December 2016 to February 2019 were identified, and included in the study if they had both CA19-9 and CEA tests available. ctDNA, mismatch repair testing by IHC and NGS tissue genetic testing results were used to categorize patients into BRAF V600E MSS, MSI-High, RAS mutant MSS and RAS/RAF wild type CRCs. A total of 85 patients were included in the study. Among them, 7 patients were BRAF V600E MSS, 6 were MSI-High, 20 were RAS mutant MSS and 52 were RAS/RAF wild type.
When patients had multiple records of CA19-9/CEA, the maximum ratios for each patient was first identified, and summarized as mean (standard deviation) and median (range) for the entire cohort and by mutation types. Kruskal-Wallis test was used to compare it between mutation types and the pairwise P values were adjusted for multiple comparisons with Holm method. For sensitivity analysis, the same analysis was repeated for the mean and median ratio of each patient. All tests were two-sided with alpha level set at 0.05 for statistical significance. R3.4.2 was used for statistical analysis. Ethical approval of the study was obtained by Mayo Clinic Institutional Review Board (IRB) for this retrospective analysis. It is exempt from consent since it is a retrospective database study.
Results
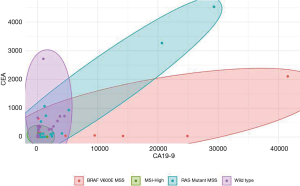
Figure 1 and Table 1 summarize the results of our study. BRAF-V600E MSS CRC patients had a discordantly profound elevation in CA-19-9 levels as opposed to the CEA levels. This is demonstrated in Figure 1, with BRAF-V600E MSS patients having the widest ellipse in contrast to the MSI-High patients having the smallest ellipse. The box plots of CA19-9/CEA ratios demonstrated in Figure 2 clearly distinguish the patients with BRAF V600E MSS and patients with MSI-High CRC from RAS mutant MSS and wild type tumors. All the ratios including mean, median and max ratios were highest in BRAF V600E MSS patients and lowest in MSI-High patients. The preliminary results of the study have been accepted as an abstract for ASCO 2019.
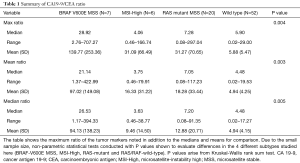
Full table
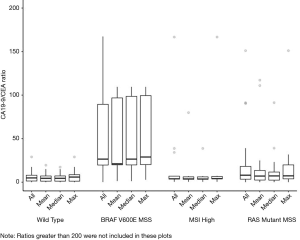
Furthermore, patients in the BRAF V600E MSS subset had the highest CA19-9/CEA ratio among all mutation types when maximum, mean and median ratio of each patient were analyzed. The median of maximum CA-19-9/CEA ratio was highest in BRAF-V600E MSS patients [28.92 (range, 2.76–707.27)] and least in the MSI-High subset of patients [4.06 (range, 0.46–166.74)], P value of 0.004. Similarly, the median of mean CA-19-9/CEA ratio for BRAF V600E MSS tumors was 21.14 (range, 1.37–422.99) versus 3.75 (range, 0.46–79.91) for MSI-High patients, clearly distinguishing them from RAS mutant MSS and wild type patients. The summary of CA19-9/CEA ratio for each mutation type is shown in Table 1. The pairwise comparison (Table 2) shows that, the difference in ratio between BRAF and wild type was statistically significant in all three analyses.
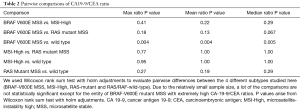
Full table
Discussion
The biological evolution of CRC involves multiple molecular pathways, the most important of which are chromosomal instability, microsatellite instability and CpG island methylator phenotype pathways (3). Chromosomal instability positive CRC have mutations in oncogenes like KRAS, PIK3CA and tumor suppressor genes like APC, TP53, SMAD4, etc. (4). Development of CRC with inactive DNA mismatch repair system characterizes the microsatellite instability (MSI) pathway. The third pathway, CpG island methylator phenotype pathway occurs due to hypermethylation of CpG sequences with BRAF oncogene as the driver mutation (3).
BRAF V600E positive CRCs account for only 5–8% of CRC, but have a very poor prognosis, especially in BRAF V600E microsatellite stable (MSS) patients (5-9). Recently targeted therapy approaches utilizing anti-EGFR alongside BRAF-inhibitors are now included in the guidelines for after failure of first line therapy. Therefore, prompt identification of BRAF V600E MSS tumors plays an important role in the course of disease in these patients. National Comprehensive Cancer Network (NCCN) recommends that all patients with metastatic CRC should get mutational testing of the tumor for BRAF mutations (10).
On the other hand, various studies have shown that patients with MSI-High CRC do not respond as well to the standard chemotherapy regimens, but benefit tremendously from immune check point inhibitor therapy (2,11,12). MSI testing or mismatch repair (MMR) testing has been the standard method for detecting microsatellite instability in CRC patients.
Traditionally, other than mutational analysis, immunohistochemistry testing and/or next-generation sequencing, there is no accurate way of identifying these patients. Delays occur due to unavailability or inadequacy of tissue for genetic testing requiring repeat biopsies. Additionally, while this is standard recommended practice, mutational testing is still not available or affordable in all parts of the world.
Carbohydrate antigen 19-9 (CA19-9) and CEA are two important tumor markers which are routinely used in GI malignancies. While CEA is usually the main tumor marker utilized in CRC, CA-19-9 also is of value and was actually initially developed in CRC cell lines. Elevated levels of CEA and CA19-9 are associated with advanced colorectal neoplasia and CRC (13). A study by Zhang et al. elaborated the importance of using combination of both CEA and CA19-9 in improving the sensitivity of diagnosing CRC rather than using the tumor markers alone (14). Several studies have elaborated the importance of serum CA19-9 and CEA as prognostic markers for patients with metastatic CRC (15,16). CA-19-9 repeatedly is associated with worse prognosis.
Our study innovatively describes the importance of the ratio of CA19-9/CEA in identifying patients with BRAF V600E MSS and MSI-High CRC. The results were intriguing with the ratio being bizarrely high in BRAF V600E MSS patients and lowest in MSI-High patients, thus differentiating them from other subset of patients with RAS MSS and wild type tumors. The ratio of CA19-9/CEA therefore helps analyze and predict the mutational status of CRC patients, much prior to the availability of results of molecular analysis of the tissue sample. In the clinical setting, it can take up to 10–14 days for the results of molecular testing, whereas for tumor markers like CA19-9 and CEA, the results are available within a few hours. Having the first line BRAF-V600E mutant clinical trials, early identification of these patients would be the key. Furthermore, even in the absence of clinical trials, patients with BRAF-V600E metastatic CRC with good performance status require triplet chemotherapy regimen with or without an anti-vascular endothelial growth factor (VEGF) agent like bevacizumab. Without the results of molecular testing, often these patients may be started on a doublet chemotherapy with or without an anti-VEGF from which they would not derive benefit. Thus, our study lays the foundation for using two simple lab tests to predict the mutational type of patients with CRCs. As noted earlier, if validated by a larger study, it could be particularly of value in parts of the world where still mutational testing is either not available or affordable.
Limitations
Limitations of our study are the relatively small sample size and the retrospective nature of the study. Despite these, given the significant differences observed, our study is more than hypothesis generating.
Conclusions
To date, this is the first report utilizing the ratio of tumor markers CA-19-9/CEA as predictive rather than just prognostic markers. It clearly identifies BRAF-V600E MSS and the MSI-High CRC patients from other subsets, with BRAF-V600E MSS subset having the highest ratio of CA19-9/CEA versus MSI-High CRC patients having the lowest ratio. In conclusion, our data supports the predictive role of ratio of CA19-9 and CEA (CA19-9/CEA) in differentiating BRAF-V600E MSS as well as MSI-High CRC patients. This could be particularly of value in parts of the world where still mutational testing is either not available or affordable.
Acknowledgments
Funding: We are deeply indebted to the Division of Surgery for their support for funding the statistical analyses for this study.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo.2019.12.08). PM Kasi: Advisory Board/Consultancy: Taiho and Ipsen (to institution); Research Funding/Clinical Trials: AAA (to institution), Array BioPharma (to institution), BMS (to institution) and Celgene (to institution). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical approval of the study was obtained by Mayo Clinic Institutional Review Board (IRB) for this retrospective analysis (No. 17-005846). It is exempt from consent since it is a retrospective database study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thiel A, Ristimäki A. Toward a Molecular Classification of Colorectal Cancer: The Role of BRAF. Front Oncol 2013;3:281. [Crossref] [PubMed]
- Fabrizio DA, George TJ Jr, Dunne RF, et al. Beyond microsatellite testing: assessment of tumor mutational burden identifies subsets of colorectal cancer who may respond to immune checkpoint inhibition. J Gastrointest Oncol 2018;9:610-7. [Crossref] [PubMed]
- Colussi D, Brandi G, Bazzoli F, et al. Molecular pathways involved in colorectal cancer: implications for disease behavior and prevention. Int J Mol Sci 2013;14:16365-85. [Crossref] [PubMed]
- Pino MS, Chung DC. The chromosomal instability pathway in colon cancer. Gastroenterology 2010;138:2059-72. [Crossref] [PubMed]
- Sanz-Garcia E, Argiles G, Elez E, et al. BRAF mutant colorectal cancer: prognosis, treatment, and new perspectives. Ann Oncol 2017;28:2648-57. [Crossref] [PubMed]
- Ogino S, Shima K, Meyerhardt JA, et al. Predictive and prognostic roles of BRAF mutation in stage III colon cancer: results from intergroup trial CALGB 89803. Clin Cancer Res 2012;18:890-900. [Crossref] [PubMed]
- Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res 2005;65:6063-9. [Crossref] [PubMed]
- Fariña-Sarasqueta A, van Lijnschoten G, Moerland E, et al. The BRAF V600E mutation is an independent prognostic factor for survival in stage II and stage III colon cancer patients. Ann Oncol 2010;21:2396-402. [Crossref] [PubMed]
- Lochhead P, Kuchiba A, Imamura Y, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst 2013;105:1151-6. [Crossref] [PubMed]
- Engstrom PF, Arnoletti JP, Benson AB 3rd, et al. NCCN Clinical Practice Guidelines in Oncology: colon cancer. J Natl Compr Canc Netw 2009;7:778-831. [Crossref] [PubMed]
- Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219-26. [Crossref] [PubMed]
- Jover R, Zapater P, Castells A, et al. The efficacy of adjuvant chemotherapy with 5-fluorouracil in colorectal cancer depends on the mismatch repair status. Eur J Cancer 2009;45:365-73. [Crossref] [PubMed]
- Kim NH, Lee MY, Park JH, et al. Serum CEA and CA 19-9 Levels are Associated with the Presence and Severity of Colorectal Neoplasia. Yonsei Med J 2017;58:918-24. [Crossref] [PubMed]
- Zhang SY, Lin M, Zhang HB. Diagnostic value of carcinoembryonic antigen and carcinoma antigen 19-9 for colorectal carcinoma. Int J Clin Exp Pathol 2015;8:9404-9. [PubMed]
- Sisik A, Kaya M, Bas G, et al. CEA and CA 19-9 are still valuable markers for the prognosis of colorectal and gastric cancer patients. Asian Pac J Cancer Prev 2013;14:4289-94. [Crossref] [PubMed]
- Wang J, Wang X, Yu F, et al. Combined detection of preoperative serum CEA, CA19-9 and CA242 improve prognostic prediction of surgically treated colorectal cancer patients. Int J Clin Exp Pathol 2015;8:14853-63. [PubMed]


