Retroperitoneal nodular fasciitis: a benign etiology on the differential diagnosis of malignant gastric outlet obstruction
Introduction
Gastric outlet obstruction (GOO) has a wide differential diagnosis including both benign and malignant conditions. There is a paucity of epidemiological data, however, some studies have found between 50% to 80% of GOO cases have been secondary to malignancy (1,2). Malignancy-related GOO is typically associated with patients presenting with primary gastrointestinal cancers of the stomach, duodenum, pancreas and biliary tract, however, metastatic lesions have also been reported (3). About 15% to 25% of patients with pancreatic cancer can present with GOO, therefore malignancy is a common consideration when GOO is present (3). Benign causes of GOO include peptic ulcer disease, pancreatitis, caustic ingestions, Crohn’s disease and benign tumors such as lipomas and stromal tumors (4).
Nodular fasciitis is a relatively rare, benign and proliferative lesion that consists of spindle or fibroblast-like cells. It is typically found in the subcutaneous fat or fascia of the head and neck, upper extremities and trunk (5). Nodular fasciitis is not commonly found in the abdomen, including the RP space, nor included on the differential diagnosis for GOO. Regardless of location, nodular fasciitis can sometimes be difficult to distinguish from malignant lesions and the diagnosis can only be rendered by a synthesis of history, physical exam, imaging and histopathology findings.
Case report
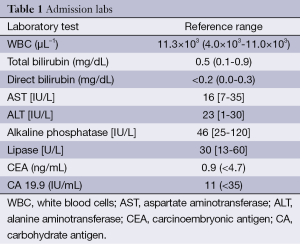
A 45-year-old Caucasian male with history of cholelithiasis presented with several weeks of increasing post-prandial epigastric and right upper quadrant (RUQ) abdominal pain along with occasional vomiting. He had no personal or family history of malignancy. His abdominal exam was significant for mild epigastric and RUQ abdominal pain and negative Murphy’s sign. Admission labs are included in Table 1 and significant only for a slight leukocytosis of 11.3×103/µL (reference range, 4.0×103-11.0×103/µL) without a left shift. Abdominal ultrasound re-demonstrated multiple mobile gallstones without evidence of acute cholecystitis.
Full table
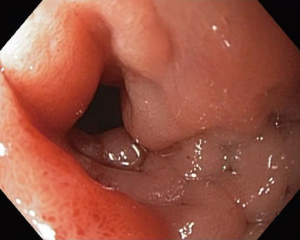
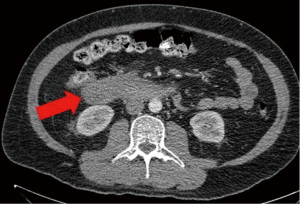
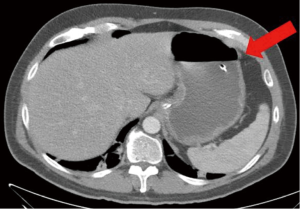
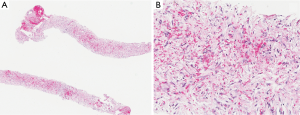
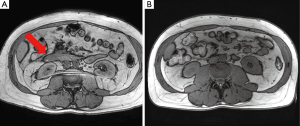
The patient had a presumed diagnosis of biliary colic and was taken to the operating room for a laparoscopic cholecystectomy. However, upon entering the peritoneal cavity, a large paraduodenal mass was visualized that was concerning for malignancy and the procedure was aborted. Computed tomography (CT) of the abdomen and pelvis with and without contrast revealed a large, heterogeneous RP mass measuring 5.2 cm × 3.2 cm (in axial dimension) adjacent to the third portion of the duodenum (Figure 1) causing partial obstruction and without adjacent lymphadenopathy (LAD). This duodenal obstruction led to a dilated and fluid-filled stomach (Figure 2). Subsequent esophagogastroduodenoscopy (EGD) showed extraluminal narrowing of the second and third portion of the duodenum with no ulcerations (Figure 3). A CT-guided core biopsy of the mass showed a low grade spindle cell proliferation with tissue culture-like appearance, microcystic areas and extravasated red blood cells (Figure 4). No necrosis or cytologic atypia was identified. Immunostains were negative for AE1/AE3, S100, CD117, CD34 and Alk-1, which was consistent with a diagnosis of nodular fasciitis.
The patient’s symptoms spontaneously resolved during his hospitalization and did not require any additional surgical or endoscopic interventions. Positron emission tomography-computed tomography (PET-CT) at 3 weeks post-presentation showed no evidence of LAD or metastatic disease with the mass decreased in size to 4.0 cm × 1.5 cm, reduction in extrinsic duodenal compression and no increased metabolic activity. MRI/MRCP at 3 months showed no RP mass (Figure 5). The patient eventually underwent a laparoscopic cholecystectomy five months after the initial mass was found with no complications during or after the procedure. The patient was doing well and asymptomatic at his follow-up visit 2 weeks post-surgery.
Discussion
To our knowledge, this is the first case report of GOO secondary to retroperitoneal (RP) nodular fasciitis. Patients with nodular fasciitis typically present between 20 and 40 years old with a rapidly growing mass on the extremities (upper or lower) or trunk, but there are also reports in the head and neck, mouth and mesentery (5-8). Nodular fasciitis has not been commonly reported in the RP space, especially in the paraduodenal location seen in our patient. In addition to the size and appearance of the mass on the initial CT, the location within the RP space was also concerning for malignancy as 70% to 80% of all primary RP neoplasms are malignant (9).
Nodular fasciitis is considered to be a reactive fibroblastic growth in response to some form of trauma or inflammation, although the inciting event is not readily identifiable in most patients. Our patient’s inciting event may have been related to his history of cholelithiasis and biliary colic. He did not appear to have acute cholecystitis based on physical exam, laboratory studies or imaging.
Diagnosis of nodular fasciitis can be challenging since the lesions can resemble malignant tumors such as sarcomas both on imaging (e.g., CT and MRI) and histopathology. Unfortunately, many of the findings associated with nodular fasciitis on CT or MRI are nonspecific and are varied based on the histologic composition of the lesion (6). Histopathology can show spindle or fibroblast-like cells that are arranged in a way that resembles a “tissue culture appearance” as it did in our patient (5). The differential diagnoses for a spindle cell lesion (along with corresponding immunostains) in the RP space include spindle cell carcinoma (AE1/AE3), melanoma (S100) and mesenchymal lesions such as gastrointestinal stromal tumor (CD117), solitary fibrous tumor (CD34), smooth muscle tumors (actin, desmin), inflammatory myofibroblastic tumor (Alk-1) and fibromatosis (beta-catenin) (5,8). Nodular fasciitis has been reported to stain positive for α-SMA, CD10, DGP9.5, calponin and vimentin, however the presence of these stains is relatively non-specific and still a developing area of research (5,8).
Depending on the rate of growth and patient’s symptoms, nodular fasciitis can be surgically resected or observed since lesions can spontaneously regress (5). Our patient began showing symptomatic improvement within 1 week and radiographic improvement on PET-CT within 3 weeks of initial presentation. In addition to this reduction in size, the PET-CT made malignant GOO less likely by showing neither increased metabolic activity nor evidence of metastatic disease. Therefore, the patient was managed conservatively with observation and complete resolution of the RP mass was verified with MRI/MRCP performed three months after the initial presentation.
Malignant etiologies of GOO, especially in the RP space, continue to be more prevalent than benign causes. Nodular fasciitis represents an extremely rare cause of GOO, albeit an important one to consider when evaluating potentially malignant versus benign etiologies. Clinicians should be cognizant of its histopathological findings and closely review potential cases with a pathologist.
Acknowledgements
Informed consent was granted by the patient prior to completion of this case report. This case report was accepted as a significantly shorter abstract to the ACG 2014 meeting. The current version submitted has significantly more detail compared with the abstract version.
Authors’ contributions: CA Kistler designed and drafted the manuscript, performed the literature search and is the corresponding author. W Jiang provided the pathology slides and reviewed the manuscript. RM Coben assisted in drafting and reviewing the manuscript and is the author guarantor.
Disclosure: The authors declare no conflict of interest.
References
- Chowdhury A, Dhali GK, Banerjee PK. Etiology of gastric outlet obstruction. Am J Gastroenterol 1996;91:1679. [PubMed]
- Shone DN, Nikoomanesh P, Smith-Meek MM, et al. Malignancy is the most common cause of gastric outlet obstruction in the era of H2 blockers. Am J Gastroenterol 1995;90:1769-70. [PubMed]
- Tendler DA. Malignant gastric outlet obstruction: bridging another divide. Am J Gastroenterol 2002;97:4-6. [PubMed]
- Appasani S, Kochhar S, Nagi B, et al. Benign gastric outlet obstruction--spectrum and management. Trop Gastroenterol 2011;32:259-66. [PubMed]
- Shiga M, Okamoto K, Matsumoto M, et al. Nodular fasciitis in the mesentery, a differential diagnosis of peritoneal carcinomatosis. World J Gastroenterol 2014;20:1361-4. [PubMed]
- Dinauer PA, Brixey CJ, Moncur JT, et al. Pathologic and MR imaging features of benign fibrous soft-tissue tumors in adults. Radiographics 2007;27:173-87. [PubMed]
- Shimizu S, Hashimoto H, Enjoji M. Nodular fasciitis: an analysis of 250 patients. Pathology 1984;16:161-6. [PubMed]
- Morgen EK, Carter P, Weinreb I, et al. Immunohistochemistry in nodular fasciitis of the head and neck. Pathology 2013;45:432-3. [PubMed]
- Rajiah P, Sinha R, Cuevas C, et al. Imaging of uncommon retroperitoneal masses. Radiographics 2011;31:949-76. [PubMed]