Liver regeneration following repeat SBRT
Introduction
Regeneration is a well-established response to liver injury. The kinetics of liver regeneration (LR) is primarily documented in surgical series following partial hepatectomy, the current gold standard for treatment of oligometastases. Given the emerging role of stereotactic body radiation therapy (SBRT) as a treatment option, it is important to review regeneration dynamics in irradiated livers. There is scant literature documenting LR after liver radiotherapy (RT). Herein, we document the first case of LR following repeat right hepatic lobe SBRT with demonstrable contralateral lobe hypertrophy.
Case description
A 58-year-old woman with previous right breast cancer presented with a metachronous, metastatic left breast cancer. Following prolonged systemic therapy and resolution of extrahepatic disease, consolidative SBRT was performed on three unresectable liver lesions <3 cm. Over 31 months, an additional three rounds of SBRT were performed on new lesions, predominantly in the right lobe (Table 1).
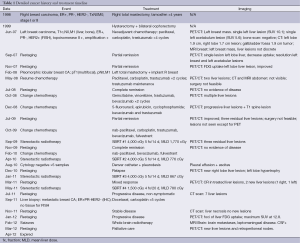
Full table
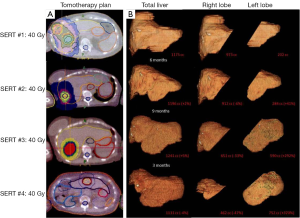
For treatment planning, the patient was immobilized in supine position. A 4-D CT scan was performed to account for respiratory motion of the liver targets. PET-positive gross tumor volumes (GTV) were contoured and an internal target volume was constructed using 4-D data sets. A 7 mm planning target volume (PTV) margin was utilized to account for daily setup error. The primary liver constraint was 700 cc <15 Gy. Plans were not optimized to intentionally spare the left hepatic lobe. Following each treatment, PET/CT confirmed a complete response in treated lesions. Liver function remained within normal limits following each course of therapy. Treatment plans are shown (Figure 1A).
Following SBRT #2, the patient developed a right pleural effusion, dull chest wall pain, and ascites. Cytology studies were negative. Following SBRT #3, she developed striking left hepatomegaly with overlying skin telangiectasias and mild tenderness without icterus or ascites. She eventually succumbed to leptomeningeal disease.
Given the profound clinical and radiographic liver contour changes, CT-based liver volumetry was performed retrospectively (Figure 1B). Retraction of the treated lobe was significant with a near 50% volume reduction. Compensatory contralateral lobe hypertrophy was noted with a 320% volume increase. The overall liver volume remained stable, within ±5% of baseline.
Discussion
Post-hepatectomy regeneration
Guideline criteria must be met prior to hepatectomy to maintain low post-operative morbidity. Surgeons must preserve two contiguous hepatic segments, adequate vascular and biliary flow, and a >20% future liver remnant (FLR) (1). Cirrhotic patients generally require 40% FLR volumes due to impaired regenerative capacity (2). In patients with inadequate predicted FLR volume, preoperative portal vein embolization (PVE) has been employed to induce hypertrophy of the FLR with excellent results (3). Adequate FLR volume is generally achieved 3-4 weeks after PVE. This technique raises concern as tumor progression in non-embolized segments has been observed. It is unknown if PVE stimulates tumor growth or redirects metastases to the previously unaffected lobe (4).
After hepatectomy, portal pressures increase and flow to hepatic tissue is enhanced, increasing hepatocyte sensitivity to hepatotrophic factors. Endothelial cell proliferation lags but results in feedback regulation to maintain liver volume homeostasis. In healthy livers, total volume recovery is complete within 2 to 6 months. Functional recovery occurs within 3 weeks (2).
Recurrence after partial hepatectomy for oligometastases is common. Interest is growing in repeat hepatectomy to “reset the oncological clock” as an equal survival benefit is observed with subsequent resections (5). Unfortunately, only 20% of patients are candidates for initial hepatic resection, and only 5-10% of those patients are candidates for repeat hepatectomy due to significantly increased surgical difficulty (6). In our patient, two surgical groups declined hepatectomy due to inability to visualize the lesions on CT or MRI.
Liver SBRT an emerging treatment option
The liver was traditionally considered a radiosensitive organ. The most worrisome complication, radiation-induced liver disease (RILD), is a syndrome of ascites, transaminitis, and anicteric hepatomegaly occurring at whole liver doses of >30-35 Gy. Radiotherapeutic advancements have provided more opportunity for partial liver RT and quantitative evaluation of dose-volume effects. Retrospective series of partial liver RT demonstrate that liver tolerance is dependent on pre-treatment Child-Turcotte-Pugh (CTP) score or pre-existing viral hepatopathy, and exhibits a clear volume effect (7). These studies validated the safety of partial liver RT if adequate liver volume is preserved. Stemming from these findings, there has been growing interest in SBRT for treatment of both primary and metastatic liver lesions. Several phase I/II trials have demonstrated excellent local control with few grade 3 toxicities (8-10). RILD risk increases with increased CTP score (11). In select patients with liver confined disease, SBRT may impact survival. Phase III studies are warranted for validation of this approach.
Does RT affect hepatic regenerative capacity?
In contrast to other organs with regenerative potential (i.e., bone marrow, skin, intestine) LR is not dependent on a few progenitor cells. In LR, terminally differentiated cells proliferate at various rates to reconstitute liver volume. In theory, integral dose to the surrounding liver parenchyma could impede LR and decrease post-treatment liver volumes. In fact, animal studies show delayed restoration of liver mass following low dose whole liver RT (12) and increased radiosensitivity of the rat liver following partial hepatectomy (13). In addition, a transient reduction in liver volume of ~20% was reported following a liver SBRT dose escalation trial at 3 months and improved to a ~10% volume reduction at 1 year (14,15). To the contrary, continuously irradiated regenerating rat livers accumulated chromosomal aberrations, however, complete LR occurred within 1 week (16). Inadequate LR following RT would limit retreatment options. The presented case demonstrates profound contralateral lobe hypertrophy following repeat SBRT and maintenance of a steady state liver volume.
Histologic changes after focal liver RT show a dose-volume effect
Histologic evaluation of irradiated liver demonstrates a dose-volume effect. In whole irradiated livers with RILD, a distinct pattern of veno-occlusive disease (VOD) is observed (17). Following focal RT in animal livers, sharply demarcated areas of radiation damage retracted and developed fibrosis over weeks to months (18). In patients treated with SBRT, three zones of injury were identified: a central necrotic zone; a repopulation zone with fibrosis, granulation tissue, and regenerating hepatocytes; and a peripheral zone of VOD (14). These three regions were surrounded by normal hepatic parenchyma, presumably with full regenerative capacity. Clinicopathologic correlation is observed in the presented case in that treated areas developed fibrosis, while areas receiving low-doses retained regenerative capacity.
Conclusions
Local treatment may confer a survival benefit for select patients with oligometastatic liver disease. Hepatectomy is the current gold standard for operable patients; however, other ablative techniques may accomplish a similar result, allowing for treatment of an increased population of patients. Our case indicates that repeat liver SBRT can be delivered safely to individual patients and that compensatory contralateral lobe hypertrophy is observed to maintain a functional liver volume. Retraction fibrosis is seen in areas receiving high radiation doses. LR dynamics following SBRT appear to mimic those documented in surgical series. As is inherent to a single case report, these results should not be considered generalizable and caution should be observed in patients with pre-existing hepatopathy or absence of observable hypertrophy following SBRT. Future studies are warranted.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Charnsangavej C, Clary B, Fong Y, et al. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol 2006;13:1261-8. [PubMed]
- Nagasue N, Yukaya H, Ogawa Y, et al. Human liver regeneration after major hepatic resection. A study of normal liver and livers with chronic hepatitis and cirrhosis. Ann Surg 1987;206:30-9. [PubMed]
- Hemming AW, Reed AI, Howard RJ, et al. Preoperative portal vein embolization for extended hepatectomy. Ann Surg 2003;237:686-91; discussion 691-3. [PubMed]
- de Graaf W, van den Esschert JW, van Lienden KP, et al. Induction of tumor growth after preoperative portal vein embolization: is it a real problem? Ann Surg Oncol 2009;16:423-30. [PubMed]
- Lam VW, Pang T, Laurence JM, et al. A systematic review of repeat hepatectomy for recurrent colorectal liver metastases. J Gastrointest Surg 2013;17:1312-21. [PubMed]
- Elias D, Lasser P, Hoang JM, et al. Repeat hepatectomy for cancer. Br J Surg 1993;80:1557-62. [PubMed]
- Dawson LA, Ten Haken RK. Partial volume tolerance of the liver to radiation. Semin Radiat Oncol 2005;15:279-83. [PubMed]
- Lee MT, Kim JJ, Dinniwell R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol 2009;27:1585-91. [PubMed]
- Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009;27:1572-8. [PubMed]
- Schefter TE, Kavanagh BD, Timmerman RD, et al. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys 2005;62:1371-8. [PubMed]
- Méndez Romero A, Wunderink W, Hussain SM, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase i-ii study. Acta Oncol 2006;45:831-7. [PubMed]
- Albert MD, Bucher NL. Latent injury and repair in rat liver induced to regenerate at intervals after x-radiation. Cancer Res 1960;20:1514-22. [PubMed]
- Geraci JP, Mariano MS. Radiation hepatology of the rat: the effects of the proliferation stimulus induced by subtotal hepatectomy. Radiat Res 1994;140:249-56. [PubMed]
- Olsen CC, Welsh J, Kavanagh BD, et al. Microscopic and macroscopic tumor and parenchymal effects of liver stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2009;73:1414-24. [PubMed]
- Stinauer MA, Diot Q, Westerly DC, et al. Fluorodeoxyglucose positron emission tomography response and normal tissue regeneration after stereotactic body radiotherapy to liver metastases. Int J Radiat Oncol Biol Phys 2012;83:e613-8. [PubMed]
- Fabrikant JI. Cell proliferation in the regenerating liver and the effect of prior continuous irradiation. Radiat Res 1967;32:804-26. [PubMed]
- Reed GB Jr, Cox AJ Jr. The human liver after radiation injury. A form of veno-occlusive disease. Am J Pathol 1966;48:597-611. [PubMed]
- Hakim R, Zervas NT, Hakim F, et al. Initial characterization of the dosimetry and radiology of a device for administering interstitial stereotactic radiosurgery. Neurosurgery 1997;40:510-6; discussion 516-7. [PubMed]


