Impact of the immune system and immunotherapy in colorectal cancer
Introduction
The immune system has a complex and multi-faceted role in cancer, affecting all aspects of the disease from tumorigenesis to treatment. Immune cells can act both as suppressors of tumor initiation and progression, as well as promoters of proliferation, infiltration, and metastasis. Within the tumor microenvironment, various immune cells have been described in virtually all tumor types with the exact composition of immune cells depending on the tumor origin, location, and individual characteristics of the patient. Both innate immune cells [macrophages, mast cells, neutrophils, dendritic cells (DCs), myeloid derived suppressor cells, and natural killer (NK) cells] and adaptive immune cells (T and B lymphocytes) are present and interact with the tumor via direct contact or through chemokine and cytokine signaling which shapes the behavior of the tumor and its response to therapy (Figure 1). Increased understanding of the immune microenvironment of tumors has allowed for an explosion of the identification of novel immune-based biomarkers and the development of new agents that target immune pathways for therapy. This review is aimed at outlining the numerous roles that the immune system plays in cancer and the treatments that take advantage of our growing understanding of the immune system, with a particular emphasis on colorectal cancer (CRC).
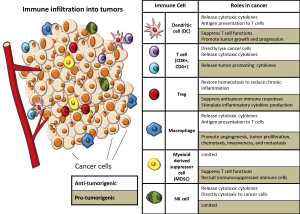
Anti-tumor immune responses
The immune responses to tumors share a number of similarities with the host immune response to infections and foreign antigens. Innate immune cells such as NK cells, macrophages and DCs can respond to both microbe-associated molecular patterns or to inflammatory signals generated by damaged tissues. Recognition by innate immune cells initiates an inflammatory cascade that leads to antigen presentation by DCs and macrophages to T cells, activating an adaptive immune response (1). More specifically, in cancer, the innate immune system recognizes tumor specific antigens on the surface of cancer cells in a manner similar to the recognition of non-self-pathogens. Innate immune cells, such as NK cells, recognize the lack of MHC-I surface molecules on cancer, engage in active killing of these cells and then recruit other inflammatory cells through their cytokine production (2). Recruited monocytes, namely macrophages and DCs, phagocytose tumor cells and then present tumor-associated antigen on their surface (3) which activates a specific cytolytic T cell response that is directed against the tumor. Then, like a pathogen induced immune response, these specific effector T cells clonally expand and travel to the tumor to eradicate it from the body (4). However, just like microbes that cannot be controlled or are chronic, cancer cells undergo a selection process for cells that have the ability to evade the immune system by acquiring several key properties including decreased immunogenicity, expression of a highly immunosuppressive microenvironment, and the ability to stimulate a supportive immune microenvironment rich in factors that support nutrient acquisition, angiogenesis and matrix remodeling (1). We discuss in more detail these properties of tumors that allow them to evade the anti-tumor immune response in the following sections.
Immunosurveillance
A number of immune cells have the ability to directly and indirectly kill cancer cells. In fact, it has been found that immune cells patrol the body monitoring for the altered cells that become cancer in a process known as “immunosurveillance”. Through immunosurveillance, the body can effectively recognize and eliminate cancerous cells prior to them causing harm (5). Evidence that immunosurveillance plays a critical role controlling the development of cancer comes from patients who have been immunosuppressed such as transplant recipients or patients with advanced HIV infection who have a higher risk of a number of cancers, including colon and pancreatic cancer, compared to normal, uninfected individuals (6,7). One of the key cell types involved in immunosurveillance is NK cells which can cause direct cytotoxicity of cancer cells, which frequently do not express any MHC-I class alleles, making them susceptible to NK killing (8), as well as release cytotoxic granules containing perforin and granzyme B (9). Other immune cells are also involved in the killing of cancer cells, but have more complex roles in which they have also been described to promote tumor growth, depending on the context (10). These other anti-tumor immune cells include but are not limited to, CD8+ T cells with can directly lyse cancer cells and produce cytokines that promote a cytotoxic response such as interferon gamma (IFN-γ) (11), CD4+ Th1 cells which can stimulate production and function of cytotoxic T lymphocytes (CTLs) and produce toxic cytokines including IFN-γ and interleukin 4 (IL-4) (12), CD4+ Th17 cells which activate CTLs (13,14), CD4+ regulatory T (Treg) cells which can suppress chronic inflammation (15), and neutrophils which are involved in direct cytotoxicity and regulation of CTL responses (14,16). Macrophages and DCs can also participate in the production of an anti-tumor immune response through their ability to present tumor antigens to T cells and through their response to danger and stress signals which causes the release of critical cytotoxic cytokines (17-19). These anti-tumor immune cells have been used as both prognostic markers with more anti-tumor immune responses correlating with better outcomes as well as targets for immunotherapy in which groups have sought ways to augment the anti-tumor response.
There is increasing evidence suggesting that immune cells play an important role in regulating the development of tumors in CRC. In one example, a study of 49 fresh CRC tumor samples ranging from stages II to IV found that the higher the number of activated (CD69+) and cytotoxic (CD107a+) CD8+ tumor infiltrating T lymphocytes (TILs), the higher the number of tumor antigen-reactive T cells in the blood and bone marrow. Further, the number of these activated cells inversely correlated with overall stage of the tumor. More specifically, earlier tumor stages showed higher proportions of activated CD8+ TILs. This suggests that early stage CRC may be recognized and undergo surveillance by the immune system (20).
Immunoediting and immune deficiencies in cancer
As immune cells search and destroy pre-cancerous cells, they select for tumor cells that display decreased tumor immunogenicity in a process known as immunoediting. This reciprocal relationship that immune cells have with cancer cells is defined by the “three Es of cancer immunoediting”: elimination, equilibrium, and escape (21,22). As a tumor takes root, the immune cells are gradually unable to eliminate all cancer cells but still can prevent expansion and metastasis, keeping the tumor at bay and producing a static phase known as the equilibrium phase. Over time, the dynamic interaction between the tumor and the immune system eventually results in a selection for tumor cells that can now escape the immune system leading to the development of clinically apparent tumors. Evidence for this sequence of events is supported by mouse tumor transplant data. Tumors transferred from immunodeficient mice into wild type mice can be more immunogenic than those arising from wild type mice because the tumor cells are “unedited” and do not undergo a selection process for the less immunogenic cells (23). Additionally, studies have shown that tumors arising in mice with specific immune deficiencies, including IFN-γ (24,25) and NKT cells (26), can be eliminated when transplanted into immune competent mice but grow more aggressively when transplanted into mice with the same immunodeficient genetic background (5). Additional mouse models of various types of cancer have shown that deficiencies in CD8+ CTLs, CD4+ T helper 1 (Th1) cells, or NK cells all lead to an increase in tumor incidence (27). In CRC, a study of 286 CRC tissue samples revealed that node-negative CRC had an increasing percentage of CD3+ immunoreactive areas which reduced the risk of metachronous tumors. However, in node-positive patients, CD3+ density was no longer predictive, suggesting the importance of immune evasion in CRC (28).
Pro-tumor immune responses
Suppressing the anti-tumor immune response in cancer
In addition to evading recognition by the immune system, recent experimental evidence supports the notion that tumors establish a microenvironment that actively suppresses an immune response. The first suppression mechanism utilized by tumors is the release of immunosuppressive factors such as TGF-β from cancer cells themselves to prevent CTLs and NK cells from destroying the tumor. The second mechanism involves the recruitment of immunosuppressive immune cells, such as Tregs and CD11b+Gr1+ myeloid-derived suppressor cells (MDSCs, as defined in mice) by cancer cells to evade lymphocyte-induced death (27). Tregs suppress the proliferation, cytokine expression, and activation of other T cells including CD4+ and CD8+ T cells and larger numbers of intratumoral Tregs has been correlated in numerous tumors types to poorer prognosis (29). MDSCs are a heterogeneous population of myeloid-derived cells defined as Lin-HLA-DR-CD33+ or CD11b+CD14-CD33+ in humans and consists of myeloid progenitors, immature macrophages, immature granulocytes, and immature DCs. MDSCs produce factors, such as arginase-1, which are potent suppressors of various T cell functions and thus suppress anti-tumor activities (30).
In colon cancer, a study of 64 CRC patients revealed that CRC patients had markedly increased percentages and absolute numbers of MDSCs [Lin(-/low)HLA-DR-CD11b+CD33+] in their peripheral blood when compared with healthy individuals. This increase correlated with clinical cancer stage and tumor metastasis, though not primary tumor size. A similar increase of MDSCs was also seen in the tumor tissue when compared to matched paraneoplastic tissue. Finally, in vitro studies revealed that only MDSCs from CRC patients, but not healthy donors, were able to inhibit autologous T cell proliferation (31).
The case of FoxP3+ Tregs is much more complex and varies by tumor type, stage and tissue of origin. Knowing that Tregs suppress an immune response, one would expect that they would be a poor prognostic factor as they would suppress anti-tumor immune responses, which appears to be the case in many situations (29). However, it has also been shown in several studies that Tregs can functionally restore homeostasis during chronic inflammation and reduce risk as well (15,32-34). In some solid tumors, such as ovarian carcinoma, pancreatic ductal carcinoma, and hepatocarcinoma, a large number of CD3+CD4+CD25+FoxP3+ cells correlated with poor prognosis. On the other hand, high numbers of CD3+CD25+FoxP3+ has been associated with good prognosis in follicular lymphoma, Hodgkin’s lymphoma, and head and neck cancer (35). In CRCs, the complex role of Tregs is only now being elucidated. In ulcerative colitis associated colon cancer, a study found a high frequency of FoxP3+IL-17+CD4+ Tregs in the colitic microenvironment and associated colon carcinoma. These cells were able to not only suppress T cell activation, but the IL-17+Foxp3+ Treg cells also contributed to inflammation by stimulating inflammatory cytokine production due to their release of IFN-γ and IL-2 in the colitic tissues (36). In the case of sporadic colon cancer, several studies have shown that increased frequencies of Tregs are associated with poor prognosis and an inability of the immune system to effectively respond to cancer. However, other studies have shown that a large number of intratumoral FoxP3+ Tregs correlates with a positive outcome (32). It is believed that these Tregs inhibit the local inflammatory processes that promote carcinogenesis (37).
Conditioning the tumor microenvironment
Immune cells, especially tumor-associated macrophages, have been shown to promote angiogenesis, cancer cell proliferation, and invasiveness (17,38). Tumor cells require neovasculature to supply nutrients and to clear waste. As the tumor progresses, angiogenesis is required to sustain the environment and new vessels are sprouted from existing vasculature. Before this “angiogenic switch” is turned on, necrotic tumor cell death can occur. Unlike cell death occurring through apoptosis and autophagy which generally results in phagocytosis by neighboring cells and does not elicit an immune response, necrotic cell death results in a spewing of cell contents, triggering proinflammatory signals in the local tissue, causing a recruitment of immune cells (27). These proinflammatory signals, including IL-1 and high-mobility group box 1 (HMGB1), result in both angiogenesis promotion and homing of immune cells that release additional growth factors contributing to the survival of the cancer cells (17). Innate immune cells, including macrophages, neutrophils, mast cells, and myeloid progenitors, help trigger this “angiogenic switch” and stimulate the process of new vasculature formation. The on-going signals from tumor cells, which simulate chronic inflammation, helps maintain the process (27). Immune cells also produce cytokines that work to activate transcription factors, such as NF-κB and STAT3, which promote tumor cell proliferation, growth, and survival (17). Additionally, in order for the cancer cells to continue to grow and metastasize, tumor cells must be able to invade into the peripheral area. Macrophages also contribute to this process by releasing enzymes, including metalloproteinases (MMPs) (39,40) and cysteine cathespin proteases (41), that degrade the surrounding matrix and allow invasion, eventually leading to metastasis (17,27). Other inflammatory cell types have also been implicated in supporting tumor growth. In particular, neutrophils have been shown to promote the metastatic potential of cancer cells. In one example using a UV-induced melanoma mouse model, Bald et al. found the presence of neutrophils stimulated melanoma cells to move towards endothelial cells promoting metastasis to the lung (42). Thus, from the examples above it is evident that while the immune system can protect against cancer development, it can also support the growth and metastasis of tumors through the tumors ability to co-opt the normal repair and wound healing functions of immune cells such as macrophages.
Colon cancer exhibits a number of pro-tumor inflammatory responses. In hepatocellular carcinoma (HCC) and colitis-associated CRC, high levels of IL-6 have been shown to activate STAT3 and have tumor promoting activity (17). In addition, analysis of CRC specimens have been shown to have high levels of macrophage-derived MMP-9. MMP-9 specifically degrades type IV collagen, a major component of the basement membrane, and allows metastasis to occur. The presence of high levels of MMP-9 in CRC tissue was shown to be an independent predictor of metastasis and poor outcome (43).
Chronic inflammation and cancer
In addition to promoting the growth of established tumors, chronic inflammation has been recognized as a contributor to neoplastic formation, as many of the processes such as tissue remodeling and angiogenesis found in chronic inflammatory sites are critical components in tumor development. At least 20% of cancers including pancreatic, gastric, and skin cancers have been directly linked to chronic infections (44,45). The microenvironment that is created during an inflammatory response has also been shown to initiate carcinogenesis through the production of genotoxic compounds that can damage DNA such as reactive oxygen species (ROS) (46). In addition, a number of inflammatory cytokines that can upregulate ROS and reactive nitrogen intermediates (RNI) that lead to deleterious DNA damage or activate pro-survival/proliferation pathways such as STAT3 and NFκB are present and allow damaged cells to survive (47).
As the mechanisms driving carcinogenesis are being elucidated, it has become increasingly clear that chronic inflammation is a carcinogenic process. A few examples of cancer-related chronic inflammatory diseases in the gastrointestinal system include the link of CRC to inflammatory bowel disease, gastric cancer to gastritis and ulcers, pancreatic carcinoma to pancreatitis, HCC to hepatitis and gall bladder cancer to chronic cholecystitis. Other GI malignancies linked to inflammation include anal carcinoma to chronic cervicitis and the link of oral squamous cell carcinoma to gingivitis (35). The cause of chronic inflammation in many of these situations remains unknown. However, in several cancers, specific microbial infections have been revealed to be the underlying etiology of the chronic inflammation. Perhaps the most notable is the gram-negative bacillus Helicobacter pylori which is associated with gastric cancer and has been shown both in murine models and humans to cause chronic inflammation that promotes cancer. This observation has been validated in numerous large epidemiologic studies (48). Other infections, such as hepatitis B or C, human papillomaviruses, and Bacteroides have been linked to HCC, anal, and colon cancer, respectively (17,49).
IBD-related-CRCs account for less than 2% of all CRC appearing annually. Other high-risk conditions include hereditary diseases, which may account for up to 20% of all cases. However, chronic inflammation of the colon does increase the risk of CRC to varying extents depending on a number of factors that may regulate inflammation including disease severity, duration of the disease, and proper management of the disease (47,50). Interestingly, recent evidence has pointed to intestinal inflammation driven by the microflora. When the intestine becomes overpopulated with “bad” microbes there is thought to be increased barrier disruption with a resultant increase in inflammatory and pro-tumorigenic cytokines from increased exposure to the intestinal microflora. The release of inflammatory cytokines and the ensuing immune reaction result in epigenetic changes, further recruitment of immune cells, and constant tumor-promoting signals that contribute to progression of tumor growth once the cancer is already initiated (47).
Immune cells as prognostic factors
Given the important role of the immune system in the initiation, maintenance, and progression of cancer, it is not surprising that recent studies have revealed a connection between the presence of specific immune cells and disease outcomes. Several groups have developed algorithms that quantify the presence of specific immune cells as prognostic factors. A number of cancers have been shown to have a favorable prognosis with increased infiltration of certain T cell subsets, particularly those that suggest that an individual already has a pre-existing spontaneous anti-tumor immune response. Memory T cells (CD3+CD45RO+) of the Th1 and cytotoxic types and CD8+ T cells have been shown to predict for better disease outcomes in esophageal cancer, renal cell carcinoma, and CRC, among others (32). Clinical epidemiology data has shown that patients with colon and ovarian tumors that have large numbers of CTLs and NK cells have a better prognosis than patients with fewer killer lymphocytes (27). In addition to favorable prognosis from the presence of effector cells, a number of studies have shown that the presence of mature antigen-presenting DCs, which theoretically lead to an enhanced immune response, have also been correlated with improved survival. A study of 74 non-small cell lung cancer (NSCLC) found that the presence of DC-LAMP+CD83+ mature DCs, often in tertiary lymphoid structures with DC-T cell clusters, was highly associated with good prognosis (51). Mature DCs, characterized as CD83+HLA-DR+CD40+CD86+, were also found to infiltrate colon cancer. However, the density of these DCs was found to be three times lower than seen in normal colonic mucosa and very rare in metastatic tumor tissue. In patients who had a high number of TNFα-producing TILs, a greater number of mature DCs were also observed. Thus, in many cancers, including CRC, high densities of DCs serve as a positive prognostic factor (52). While mature DCs appear to be favorable, macrophages, which in some settings have similar functions to DCs, appear to be strongly influenced by the tumor microenvironment. They are often alternatively-activated rather than cytotoxic, and produce a number of factors that influence growth and survival of tumor cells, angiogenesis, cell invasion, chemotaxis, or inhibit T cell responses (35). Thus a high number of tumor-associated macrophages is typically considered to be a poor prognostic factor (53,54).
The use of immune cells to predict tumor behavior has been an area of intense research over the past several years and in CRC in particular, several powerful predictive algorithms based on immune cells have emerged. Early studies in CRC found that the presence of CD8+ T cells, CD27-CD45RA- effector memory T cells and a Th1 gene signature had improved metastasis free and disease free survival (35,55). As these observations were repeated in large cohort studies, specific immune responses within the tumor site were found to influence clinical outcome at all stages of CRC. In fact, the type, density, and location of immune cells had a prognostic value that surpassed the UICC-TNM classification. Thus, an immune score from 0 to 4 based on the assessment of CD8+ and CD45RO+ cell densities in the center and in the invasive margins of the tumor was created. A higher score, meaning higher density of TH1/cytotoxic memory T lymphocytes in both the center and at the margin, correlates with higher disease free survival and overall survival, as well as low risk of relapse and metastasis, in CRC and is likely applicable to most human tumors, particularly those that are thought to be more sensitive to immune regulation such as melanoma (35,56,57). A worldwide harmonization effort is currently underway to confirm the utility of the immunoscore in CRC and to refine the criteria that will be used for future clinical trials.
Thus, as expected from a system as diverse as the immune system, the role of immune cells in the development, progression and treatment of tumors is very complex and not yet fully understood. The immune system, through NK cells, T cells, macrophages and DCs, helps prevent cancer by detecting and eradicating mutated cells that would become cancerous. This immunosurveillance function has been controlled or subverted by the time tumors have become clinically apparent. The goal of much of immunotherapy, as discussed in the next section, is to reawaken this anti-tumor immune response by attempting to generate de novo or more powerful anti-tumor immune responses. However, as the tumor has already managed to prevent the body’s normal anti-tumor immune response by developing powerful suppression mechanisms, strategies aimed at inhibiting immune suppressive pathways have also been surprisingly successful. Targeting the cells and pathways used by tumors to accomplish this has produced a number of recent successes that have inspired a new generation of immune-based therapeutic options.
Immunotherapy
Immunotherapy refers to therapeutic approach that harnesses the immune system to eliminate tumors. As we have described above, tumors, including colorectal tumors, employ multiple strategies to evade and suppress the immune system. Immunotherapeutic approaches have aimed at either augmenting the anti-tumor immune response through strategies such as vaccination in combination with immune stimulatory cytokines or preventing the suppression of a response through the use of checkpoint inhibitors such as the anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) antibody, ipilimumab (Figure 2). We review here recent advances in immunotherapy and current progress in applying immunotherapeutic strategies for the treatment of colorectal malignancies.
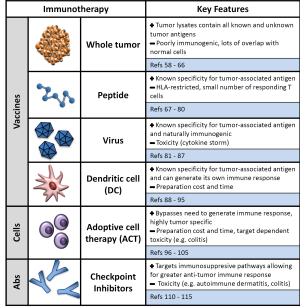
Cancer vaccines
A strategy that has been tried in multiple variations over the past two decades, cancer vaccines have had variable degrees of success eliciting an anti-tumor immune response. The concept of cancer vaccination stems from the recognition that the immune system has built in mechanisms to recognize altered self-antigens that are present on the majority of cancer cells. These antigens are often called tumor-associated antigens. Like the vaccination strategies for infectious diseases, the ultimate goal of cancer vaccination is to elicit an anti-tumor immune response that will eliminate a tumor and provide ongoing surveillance to protect against its regrowth. Numerous groups have developed and continue to develop agents that attempt to generate a productive immune response against tumors. Four major categories of vaccination agents have been explored: whole tumors, peptide antigens, DCs and viral/bacterial vaccinations.
Whole tumor vaccines
Whole tumor vaccines were the earliest of the vaccines because the vaccination material was both readily available and contained all of the known and unknown tumor associated antigens that needed to be eliminated. Thus, while there was no specific antigen identified with this approach, presumably a diverse immune response would occur that would reduce the chance that there would be tumor escape from a more specific vaccine. Typically this approach would require a sample of tumor tissue that would then be lysed or irradiated, mixed with an immune adjuvant such as alum, and then reinjected into patients (58). Autologous whole tumors have been used as cancer vaccines to induced cytotoxic anti-tumor immune responses in several cancer types including renal cell carcinoma (59), melanoma (60) and CRC (61). However, despite initial excitement for whole tumor vaccines, to date even the best trials demonstrate limited efficacy. In CRC, a randomized phase III clinical trial combining autologous whole tumor cell plus BCG vaccine was conducted by the Eastern Cooperative Oncology Group to determine whether surgical resection plus vaccination was more beneficial than resection alone in 412 stage II and III CRC patients but this study showed no significant survival or disease-free survival benefit. However, effective immune responses were associated with improved disease-free and overall survival (61).
One issue with using whole tumor vaccines is that only a small proportion of the proteins in an autologous whole tumor vaccine are specific to cancerous cells, while a vast majority of antigens in the vaccine are shared with normal cells, thus diluting the amount of tumor antigens in a whole tumor vaccine, while simultaneously supplying the antigens for stimulating an autoimmune response. Moreover, whole tumor vaccines are typically poorly immunogenic. Therefore, the immune response generated by whole tumor vaccines is generally insufficient to provide benefit to patients as evidenced by the modest results in clinical trials (62). To improve the immunogenicity of whole tumor vaccines, autologous tumor cells have been genetically modified to secrete immunostimulatory molecules such as GM-CSF and then re-administered to the patient (63). While early trials demonstrated promising results in a wide range of tumors, most of these did not result in survival benefit, though they did augment antitumor immunity (64,65). Another interesting approach to augment the immunogenicity of tumor cell vaccines utilized Newcastle disease virus (NDV)-infected irradiated tumor cells as a vaccine (66). This approach resulted in a 98% 2-year survival rate in patients with resected CRC, compared to 67% when treated with autologous tumor cells combined with BCG, suggesting that the immunogenicity of tumor cells can be altered to make them more immunostimulatory. However, the randomized phase III study of 50 patients with resectable CRC liver metastases vaccinated with NDV-infected tumor cells did not demonstrate improvement in overall survival, disease-free survival, or metastases-free survival (66). The experience with NDV-infected cells supports the notion that the immunogenicity of whole tumor cells needs to be improved for this vaccination strategy to be effective. However, as the randomized trial data demonstrated, further research into which specific agents for killing tumor cells (such as cytotoxic chemotherapeutics, ionizing irradiation, and chemical agents) can generate sufficiently immunogenic whole tumor vaccines to produce an adequate clinical anti-tumor response.
Peptide vaccines
One reason for the limited efficacy of whole tumor vaccines lies in the fact that tumor cells share the bulk of their antigens with normal cells and that the immune system is finely tuned to suppress immune responses against self-antigens. Thus in attempt to address this problem, many groups turned to peptide vaccines in order to develop an immune response against a specific known tumor antigen. Peptide-based vaccines are whole proteins or fragments of proteins typically generated from tumor-specific proteins that are administered with adjuvant. Compared to whole tumor vaccines, peptide vaccines have the potential to generate a more specific anti-tumor response by using antigens that are known to be expressed by tumor cells. Peptide vaccines have been generated for multiple tumor types including breast, prostate and pancreatic cancer (67-69). Thus far, similar to the whole cell vaccines they have shown limited efficacy in the clinical setting with many vaccines eliciting a specific response, but showing no effect on disease progression or survival benefit (62).
In CRC, multiple tumor-associated antigens have been identified and utilized for vaccination with varying success. Typically the peptides employed are designed for MHC Class I, the MHC recognized by CD8+ cytotoxic T cells. These antigens include carcinoembryonic antigen (CEA) (70), mucin-1 (71), squamous cell carcinoma antigen recognized by T cells 3 (SART3) (72), β-human chorionic gonadotropin (β-hCG) (73), Survivin-2B (74) or p53 (75), all of which have been employed as targets for immunotherapy in CRC, as well as other tumors. Peptide vaccines targeting these tumor-associated antigens have been shown to induce an antigen-specific immune response, which in some cases correlated with improved survival. For example, in one phase II trial, vaccination with the β-hCG peptide induced anti-β-hCG antibody production in 56 of 77 CRC patients (73) and, importantly, anti-β-hCG antibody induction was associated with longer overall survival. However, the majority of trials have not been able to demonstrate a correlation between an immune response and clinical outcomes. In SART3 peptide vaccine therapy, IgE-type anti-peptide antibodies were detected after vaccination; however, immunological responses were limited to patients expressing HLA-A24 (72). The results of the SART3 trial highlight one of the limitations of peptide vaccines: restricted antigen presentation due to the patient’s HLA type (76). However, peptide vaccines have other limitations including defective CD8+ CTLs due to the downregulation of certain antigens and MHC class I molecules (77), impaired DC function in patients with advanced cancer (78), and inhibitory tumor microenvironments, where immune suppressive cells such as Tregs and alternatively-activated macrophages exist (79). Given the relatively low efficacy of peptide vaccines, current strategies attempting to improve the response to peptide vaccines have focused on trying to increase the number of T cells that respond to the peptide. One strategy to do this has been to use a larger peptide to increase the number of epitopes and thus the number of T cells that may respond to a given antigen. In a phase I/II trial, 10 CRC patients were vaccinated twice with a set of 10 overlapping p53 synthetic long peptides (75). P53-specific CD4+ and CD8+ T-cell responses were observed in 9 of 10 CRC patients, and 6 of 9 tested patients maintained p53-specific T-cell reactivity for at least 6 months. New trials using peptide vaccines have also focused on administering the peptides with more effective adjuvants such as cytokines. A follow-up phase I/II trial with the p53-specific vaccine combined with interferon-alpha increased the number of interferon-gamma producing cells found in the circulation of patients with CRC (80). Thus, while early trials with peptide vaccines demonstrated low efficacy, new strategies to enhance the immune response show promise.
Viral vector vaccines
The low efficacy of peptide vaccines results from their inability to generate a productive immune response against the peptide. Thus, other groups have taken the idea of a peptide and packaged it such that it would be presented in a more pro-inflammatory way. One such strategy is through the use of a viral or bacterial vector to which the body has already developed multiple pathways to recognize. Using a recombinant virus engineered to express tumor-associated antigens takes advantage of the fact that viruses are naturally immunogenic and typically infect antigen presenting cells (specifically DCs) (81). One of the more promising approaches to augmenting immune activation combines vaccination with tumor antigens plus co-stimulatory molecules in a viral vector. The CEA/TRICOM vaccine which combines radiation, vaccination with CEA with a viral vector that expresses the three co-stimulatory molecules (TRICOM) B7.1 (CD80), intercellular adhesion molecule 1 (ICAM-1), and lymphocyte function-associated antigen 3 (LFA-3) shows excellent efficacy in a murine model of colon cancer and appears to be safe in patients (82,83). In a series of studies, CEA-specific T cell responses were observed and disease stabilization was seen in up to 40% of patients with metastatic cancer (including CRC) (84,85). In a similar strategy, another group published a phase II clinical trial in patients with metastatic CRC that examined the efficacy of chemotherapy in combination with vaccination using a nonreplicating canarypox virus (ALVAC) expressing CEA and the T-cell costimulatory molecule, B7.1 (ALVAC-CEA/B7.1). Anti-CEA-specific T cell responses were produced in 50% of patients undergoing chemotherapy and booster vaccination and objective clinical responses were observed in 40% of the patients (86,87). Current trials are underway attempting to further enhance the response by delivering the virus with tetanus toxoid and results from this strategy are still being accrued. Thus, viral vaccines produce significantly more effective responses compared to peptide vaccines, however clinical success remains elusive and is actively being pursued.
DC vaccines
As detailed knowledge of the mechanism of an immune response has become available over the past decade, it has become apparent that it is critical to provide specific essential signals to the immune system in order to produce an effective immune response against a given antigen. The three critical steps to activate a T cell are antigen presentation by MHC (signal 1), co-stimulation by an appropriate receptor-ligand pair (signal 2) and expression of key cytokines to direct the ensuing immune response. Peptide and viral vaccines depend on the use of adjuvants that stimulate an immune response themselves or the natural anti-viral immune response to produce their anti-tumor effect. The central cells for this process are DCs, which can provide all three signals for a productive anti-tumor immune response and thus, many groups have attempted to utilize DCs for vaccination (88). Many clinical trials of antigen-pulsed DCs have been completed in patients with various types of tumors, including CRC, and recent trials have begun to bear fruit (89). Several strategies for using DCs to generate an anti-tumor cytotoxic immune response have been developed. DCs have been pulsed with synthetic peptides derived from the known tumor-associated antigens, tumor cell lysates, apoptotic tumor cells or physically fused with whole tumor cells to induce efficient antitumor immune responses (Figure 2) (90-93). With respect to CRC, since CEA is a tumor-associated antigen expressed by most CRCs, many DC vaccines for CRC have utilized CEA peptides (94) or CEA-expression vectors (95). In these phase I clinical trials, the majority of vaccinated CRC patients demonstrated the induction of CEA-specific T cell responses. Furthermore, disease progression stabilized in several patients, and the vaccine was safe and well-tolerated. Despite the progress made in other cancers (89), there has not been a DC vaccine in CRC that has improved survival and the search continues for ways to improve the clinical efficacy of DC-based cancer vaccines for CRC.
Adoptive cell transfer (ACT) therapy
With the limited success of cancer vaccines for most tumor types including CRC (62), other strategies have been pursued that eliminate the need to develop a de novo immune response and circumvent the tumor-mediated suppression of an anti-tumor immune response. The most successful of these strategies has attempted to restore the cytolytic anti-tumor activity to a patient’s own T cells, thus taking advantage of the high specificity and targeting ability of T cells. This method, known as ACT therapy, extracts autologous T cells from the tumors of patients, activates them with cytokines and expands them into large numbers in vitro, all in preparation for transfer back into the patient (96). The main source for T cells for ACT comes from lymphocytes found in tumors themselves, known as TILs. It was recognized almost a decade ago that these cells are actually tumor-specific cells that had been actively suppressed by the tumor microenvironment (10,97). In ACT, the ex vivo expansion and addition of other co-stimulatory molecules and cytokines is thought to overcome tolerogenic mechanisms by selecting highly reactive T cell populations and activating them sufficiently to overcome the suppressive environment with the tumor (98). This approach has shown early and dramatic success in metastatic melanoma (97,99). However, there are several drawbacks to ACT that should be considered, including the high cost of the procedure, a potential lack of immune memory because the transfer is only activated effector cells, transient survival of the activated effector cells in patients, and the time (1-4 months) required to expand the cells. Additionally, as data from several early trials revealed, there is also a potential risk for severe adverse events (100,101).
Unfortunately, the use of TILs is currently largely limited to patients with melanoma, a reflection of the higher immunogenicity of melanoma in comparison to other cancers which often do not have infiltrating T cells with as high numbers or specificity. To address this, several groups have genetically engineered T cells to express T cell receptors (TCRs) with predetermined affinity to specific antigens to facilitate the targeting of virtually any tumor type. Several groups have shown promising data using T cells engineered to express high avidity TCRs to target tumors of various histological origins (102,103). However, these TCRs are limited to patients with the corresponding MHC haplotype. Thus, other groups have sought to engineer antibody-based chimeric antigen receptors (CARs). These receptors express a single chain variable fragment derived from a tumor antigen-recognizing monoclonal antibody fused to intracellular T cell signaling domains. With specificity provided from the antibody recognition of the antigen, these receptors can be used universally across all patients since CARs target native antigens on the surface of tumors without MHC restriction. This approach has shown early success for acute and chronic lymphoid leukemia (104,105).
Given the low number of TILs in CRC, most of the recent trials have focused on using engineered T cells. Parkhurst et al. conducted a phase I trial in colon cancer using human T cells modified to express a high avidity CEA-specific murine TCR (100). Three patients with metastatic colon cancer were treated with these engineered T cells, all of which experienced decreased serum CEA levels and one of which experienced an objective clinical response. However, all patients developed a severe transient inflammatory colitis. Severe side effects also were seen in one patient treated with Her2-specific CAR T cells for metastatic colon cancer (101). Thus, ACT has failed to demonstrate safety and efficacy in CRC patients and future studies will have to identify mechanisms that allow CAR-expressing T cells to selectively eliminate cancer cells, but leave normal tissues unaffected.
Antibody-based cancer immunotherapy
Monoclonal antibodies (mAbs), which have high specificity, have been clinically effective as cancer therapeutics for decades (106). Antibodies such as cetuximab and panitumumab which both target EGFR and bevacizumab which targets VEGF have been approved and are in current use for the treatment of CRC in the United States. Many other mAbs targeting other pathways are currently being tested in clinical trials (107). These pathways are thought to induce tumor cell death by several mechanisms, including disruption of vital signaling pathways and engaging innate immune effector mechanisms that recognize the Fc portion of the antibody via Fc receptors and induces antibody-dependent cytotoxicity through various cellular mechanisms (108). These targeted therapies are generated to block specific pathways and discussion of their effects on these signaling pathways is reviewed elsewhere (109).
Different from targeting the tumor themselves, a new class of antibodies that target the suppressive mechanisms in the tumor microenvironment has become available and have shown, in some cases, dramatic and unexpected efficacy (110). Known as checkpoint inhibitors, mAbs targeting the inhibitory immune receptors CTLA-4, programmed cell death 1 (PD-1), and PD-1 ligand (PD-L1) have produced successful results in patients with advanced melanoma and NSCLC (111-113). The success of targeting these suppressive pathways has generated tremendous excitement and trials are underway now that have the potential to radically change the concept of how we view the treatment for cancer. However, early data regarding the potential role for checkpoint inhibition in CRC suggests that anti-CTLA-4 may have limited efficacy as a single agent (114). Furthermore, preliminary studies on CRC revealed that CRC has low expression of PD-L1, suggesting that CRC may not respond to PD-1 or PD-L1 inhibition (115). Further study is warranted in the setting of CRC to determine if other therapies in conjunction with checkpoint inhibition would prove more successful.
Combined immunotherapy: key to success?
The modest success of current immunotherapeutic strategies in CRC highlights the relatively resistant nature of CRC to immune-based therapies. The mechanism underlying this resistance may have to do with an underlying connection between the immune factors that are known to drive and determine the behavior of CRC. As a tissue that is in constant contact with antigens from the microbiota, it is not surprising that CRC may have developed strong mechanisms to suppress an immune response, though these factors remain unidentified and appear not to be driven by the PD-1-PD-L1 pathway (115). However, it may be that combinations of immunotherapy or more conventional chemotherapy and radiation with immunotherapy will hold the key to developing an anti-tumor immune response. For example, one strategy might involve stimulating an immune response via vaccines, in combination with blocking inhibitory pathways such as CTLA-4 or PD-1. This would combine the strengths of a vaccine for developing an anti-tumor immune response to override mechanisms that delete anti-tumor immune cells and a checkpoint inhibitor to block inhibition of anti-tumor immune responses to overcome the suppressive microenvironment. Early clinical trials support this notion with growing evidence indicating that combined targeted therapies and simultaneous blockade of multiple immune checkpoints promotes therapeutic synergy and long-term antitumor immunity leading to improved clinical outcome in melanoma patients (116). Further, it has become increasingly evident that the efficacy of radiation and certain chemotherapies depends on the development of an immune response to the cell stress and death caused by these agents (117-119). Combining immunotherapeutics with novel immunostimulatory applications of more traditional cytotoxic agents has also shown early signs of success (120).
Conclusions
Tremendous progress has been made in understanding the role of the immune system in driving the development of cancer, including CRC. This understanding has revealed two trends that have and will continue to influence the treatment of cancer for the foreseeable future: the use of immune cells markers to predict cancer outcomes and targeting various aspects of the immune system to generate an anti-tumor immune response. With respect to the treatment of CRC, the development of the immunoscore is well underway and will likely emerge as a critical prognostic tool in the clinic. Unfortunately, effective immunotherapies in CRC remain elusive. The complex role of the GI tract, particularly the colon and small intestine, in shaping systemic immune responses is likely to account for some of the difficulties in developing effective immunotherapeutics for CRC. The most promising avenues for therapy going forward will likely be combinations of cytotoxic therapies such as chemotherapy and radiation and multiple immunotherapeutic modalities. Trials that make use of our increasing understanding of the immune system in CRC are currently underway and will no doubt continue to expand our knowledge of where immunotherapeutics fit in our current treatment paradigms.
Acknowledgements
SLS is supported by grants from the American Society for Radiation Oncology (ASTRO) and the University of California Los Angeles Clinical and Translational Science Institute (UCLA CTSI).
Disclosure: The authors declare no conflict of interest.
References
- Goldszmid RS, Dzutsev A, Trinchieri G. Host immune response to infection and cancer: unexpected commonalities. Cell Host Microbe 2014;15:295-305. [PubMed]
- Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res 2003;90:127-56. [PubMed]
- Munn DH, Cheung NK. Phagocytosis of tumor cells by human monocytes cultured in recombinant macrophage colony-stimulating factor. J Exp Med 1990;172:231-7. [PubMed]
- Van Pel A, Boon T. Protection against a nonimmunogenic mouse leukemia by an immunogenic variant obtained by mutagenesis. Proc Natl Acad Sci U S A 1982;79:4718-22. [PubMed]
- Teng MW, Swann JB, Koebel CM, et al. Immune-mediated dormancy: an equilibrium with cancer. J Leukoc Biol 2008;84:988-93. [PubMed]
- Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007;370:59-67. [PubMed]
- Di Caro G, Marchesi F, Laghi L, et al. Immune cells: plastic players along colorectal cancer progression. J Cell Mol Med 2013;17:1088-95. [PubMed]
- Zamai L, Ponti C, Mirandola P, et al. NK cells and cancer. J Immunol 2007;178:4011-6. [PubMed]
- Halama N, Braun M, Kahlert C, et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res 2011;17:678-89. [PubMed]
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012;21:309-22. [PubMed]
- Pardoll D. T cells take aim at cancer. Proc Natl Acad Sci U S A 2002;99:15840-2. [PubMed]
- Sun Q, Burton RL, Lucas KG. Cytokine production and cytolytic mechanism of CD4(+) cytotoxic T lymphocytes in ex vivo expanded therapeutic Epstein-Barr virus-specific T-cell cultures. Blood 2002;99:3302-9. [PubMed]
- Ankathatti Munegowda M, Deng Y, Mulligan SJ, et al. Th17 and Th17-stimulated CD8+ T cells play a distinct role in Th17-induced preventive and therapeutic antitumor immunity. Cancer Immunol Immunother 2011;60:1473-84. [PubMed]
- Gerrard TL, Cohen DJ, Kaplan AM. Human neutrophil-mediated cytotoxicity to tumor cells. J Natl Cancer Inst 1981;66:483-8. [PubMed]
- Erdman SE, Sohn JJ, Rao VP, et al. CD4+CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res 2005;65:3998-4004. [PubMed]
- Mittendorf EA, Alatrash G, Qiao N, et al. Breast cancer cell uptake of the inflammatory mediator neutrophil elastase triggers an anticancer adaptive immune response. Cancer Res 2012;72:3153-62. [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [PubMed]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010;141:39-51. [PubMed]
- Gallois A, Bhardwaj N. Dendritic cell-targeted approaches to modulate immune dysfunction in the tumor microenvironment. Front Immunol 2013;4:436. [PubMed]
- Koch M, Beckhove P, Op den Winkel J, et al. Tumor infiltrating T lymphocytes in colorectal cancer: Tumor-selective activation and cytotoxic activity in situ. Ann Surg 2006;244:986-92; discussion 992-3. [PubMed]
- Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002;3:991-8. [PubMed]
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004;22:329-360. [PubMed]
- Shankaran V, Ikeda H, Bruce AT, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 2001;410:1107-11. [PubMed]
- Dighe AS, Richards E, Old LJ, et al. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity 1994;1:447-56. [PubMed]
- Kaplan DH, Shankaran V, Dighe AS, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A 1998;95:7556-61. [PubMed]
- Smyth MJ, Thia KY, Street SE, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med 2000;191:661-8. [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [PubMed]
- Laghi L, Bianchi P, Miranda E, et al. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol 2009;10:877-84. [PubMed]
- deLeeuw RJ, Kost SE, Kakal JA, et al. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res 2012;18:3022-9. [PubMed]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9:162-74. [PubMed]
- Zhang B, Wang Z, Wu L, et al. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS One 2013;8:e57114. [PubMed]
- Műzes G, Molnár B, Sipos F. Regulatory T cells in inflammatory bowel diseases and colorectal cancer. World J Gastroenterol 2012;18:5688-94. [PubMed]
- Poutahidis T, Rao VP, Olipitz W, et al. CD4+ lymphocytes modulate prostate cancer progression in mice. Int J Cancer 2009;125:868-78. [PubMed]
- Erdman SE, Poutahidis T, Tomczak M, et al. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol 2003;162:691-702. [PubMed]
- Pagès F, Galon J, Dieu-Nosjean MC, et al. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene 2010;29:1093-102. [PubMed]
- Kryczek I, Wu K, Zhao E, et al. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol 2011;186:4388-95. [PubMed]
- Haas M, Dimmler A, Hohenberger W, et al. Stromal regulatory T-cells are associated with a favourable prognosis in gastric cancer of the cardia. BMC Gastroenterol 2009;9:65. [PubMed]
- Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011;475:222-5. [PubMed]
- Coussens LM, Werb Z. Matrix metalloproteinases and the development of cancer. Chem Biol 1996;3:895-904. [PubMed]
- Coussens LM, Tinkle CL, Hanahan D, et al. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 2000;103:481-90. [PubMed]
- Joyce JA, Baruch A, Chehade K, et al. Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell 2004;5:443-53. [PubMed]
- Bald T, Quast T, Landsberg J, et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 2014;507:109-13. [PubMed]
- Zeng ZS, Huang Y, Cohen AM, et al. Prediction of colorectal cancer relapse and survival via tissue RNA levels of matrix metalloproteinase-9. J Clin Oncol 1996;14:3133-40. [PubMed]
- Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res 2009;15:425-30. [PubMed]
- Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow?. Lancet 2001;357:539-45. [PubMed]
- Meira LB, Bugni JM, Green SL, et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest 2008;118:2516-25. [PubMed]
- Grivennikov SI. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol 2013;35:229-44. [PubMed]
- Hohenberger P, Gretschel S. Gastric cancer. Lancet 2003;362:305-15. [PubMed]
- Burnett-Hartman AN, Feng Q, Popov V, et al. Human papillomavirus DNA is rarely detected in colorectal carcinomas and not associated with microsatellite instability: the Seattle colon cancer family registry. Cancer Epidemiol Biomarkers Prev 2013;22:317-9. [PubMed]
- Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res 2009;29:2727-37. [PubMed]
- Dieu-Nosjean MC, Antoine M, Danel C, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 2008;26:4410-7. [PubMed]
- Schwaab T, Weiss JE, Schned AR, et al. Dendritic cell infiltration in colon cancer. J Immunother 2001;24:130-7.
- Murdoch C, Muthana M, Coffelt SB, et al. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 2008;8:618-31. [PubMed]
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer 2006;6:24-37. [PubMed]
- Camus M, Tosolini M, Mlecnik B, et al. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res 2009;69:2685-93. [PubMed]
- Galon J, Pagès F, Marincola FM, et al. The immune score as a new possible approach for the classification of cancer. J Transl Med 2012;10:1. [PubMed]
- Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J 2010;16:399-403. [PubMed]
- Blankenstein T, Coulie PG, Gilboa E, et al. The determinants of tumour immunogenicity. Nat Rev Cancer 2012;12:307-13. [PubMed]
- Jocham D, Richter A, Hoffmann L, et al. Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal-cell carcinoma after radical nephrectomy: phase III, randomised controlled trial. Lancet 2004;363:594-9. [PubMed]
- Berd D, Sato T, Maguire HC Jr, et al. Immunopharmacologic analysis of an autologous, hapten-modified human melanoma vaccine. J Clin Oncol 2004;22:403-15. [PubMed]
- Harris JE, Ryan L, Hoover HC Jr, et al. Adjuvant active specific immunotherapy for stage II and III colon cancer with an autologous tumor cell vaccine: Eastern Cooperative Oncology Group Study E5283. J Clin Oncol 2000;18:148-57. [PubMed]
- Klebanoff CA, Acquavella N, Yu Z, et al. Therapeutic cancer vaccines: are we there yet? Immunol Rev 2011;239:27-44. [PubMed]
- Tian H, Shi G, Yang G, et al. Cellular immunotherapy using irradiated lung cancer cell vaccine co-expressing GM-CSF and IL-18 can induce significant antitumor effects. BMC Cancer 2014;14:48. [PubMed]
- Salgia R, Lynch T, Skarin A, et al. Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non-small-cell lung carcinoma. J Clin Oncol 2003;21:624-30. [PubMed]
- Soiffer R, Hodi FS, Haluska F, et al. Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanoma. J Clin Oncol 2003;21:3343-50. [PubMed]
- Schulze T, Kemmner W, Weitz J, et al. Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: results of a prospective randomized trial. Cancer Immunol Immunother 2009;58:61-9. [PubMed]
- Salman B, Zhou D, Jaffee EM, et al. Vaccine therapy for pancreatic cancer. Oncoimmunology 2013;2:e26662. [PubMed]
- Beatson RE, Taylor-Papadimitriou J, Burchell JM. MUC1 immunotherapy. Immunotherapy 2010;2:305-27. [PubMed]
- Gulley JL, Drake CG. Immunotherapy for prostate cancer: recent advances, lessons learned, and areas for further research. Clin Cancer Res 2011;17:3884-91. [PubMed]
- Bilusic M, Heery CR, Arlen PM, et al. Phase I trial of a recombinant yeast-CEA vaccine (GI-6207) in adults with metastatic CEA-expressing carcinoma. Cancer Immunol Immunother 2014;63:225-34. [PubMed]
- Kimura T, McKolanis JR, Dzubinski LA, et al. MUC1 vaccine for individuals with advanced adenoma of the colon: a cancer immunoprevention feasibility study. Cancer Prev Res (Phila) 2013;6:18-26. [PubMed]
- Miyagi Y, Imai N, Sasatomi T, et al. Induction of cellular immune responses to tumor cells and peptides in colorectal cancer patients by vaccination with SART3 peptides. Clin Cancer Res 2001;7:3950-62. [PubMed]
- Moulton HM, Yoshihara PH, Mason DH, et al. Active specific immunotherapy with a beta-human chorionic gonadotropin peptide vaccine in patients with metastatic colorectal cancer: antibody response is associated with improved survival. Clin Cancer Res 2002;8:2044-51. [PubMed]
- Idenoue S, Hirohashi Y, Torigoe T, et al. A potent immunogenic general cancer vaccine that targets survivin, an inhibitor of apoptosis proteins. Clin Cancer Res 2005;11:1474-82. [PubMed]
- Speetjens FM, Kuppen PJ, Welters MJ, et al. Induction of p53-specific immunity by a p53 synthetic long peptide vaccine in patients treated for metastatic colorectal cancer. Clin Cancer Res 2009;15:1086-95. [PubMed]
- Yamada A, Sasada T, Noguchi M, et al. Next-generation peptide vaccines for advanced cancer. Cancer Sci 2013;104:15-21. [PubMed]
- Buhrman JD, Slansky JE. Improving T cell responses to modified peptides in tumor vaccines. Immunol Res 2013;55:34-47. [PubMed]
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013;14:1014-22. [PubMed]
- Shiao SL, Ganesan AP, Rugo HS, et al. Immune microenvironments in solid tumors: new targets for therapy. Genes Dev 2011;25:2559-72. [PubMed]
- Zeestraten EC, Speetjens FM, Welters MJ, et al. Addition of interferon-α to the p53-SLP® vaccine results in increased production of interferon-γ in vaccinated colorectal cancer patients: a phase I/II clinical trial. Int J Cancer 2013;132:1581-91. [PubMed]
- Larocca C, Schlom J. Viral vector-based therapeutic cancer vaccines. Cancer J 2011;17:359-71. [PubMed]
- Gameiro SR, Higgins JP, Dreher MR, et al. Combination therapy with local radiofrequency ablation and systemic vaccine enhances antitumor immunity and mediates local and distal tumor regression. PLoS One 2013;8:e70417. [PubMed]
- Gulley JL, Madan RA, Tsang KY, et al. A pilot safety trial investigating a vector-based vaccine targeting carcinoembryonic antigen in combination with radiotherapy in patients with gastrointestinal malignancies metastatic to the liver. Expert Opin Biol Ther 2011;11:1409-18. [PubMed]
- Marshall JL, Gulley JL, Arlen PM, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol 2005;23:720-31. [PubMed]
- von Mehren M, Arlen P, Tsang KY, et al. Pilot study of a dual gene recombinant avipox vaccine containing both carcinoembryonic antigen (CEA) and B7.1 transgenes in patients with recurrent CEA-expressing adenocarcinomas. Clin Cancer Res 2000;6:2219-28. [PubMed]
- Kaufman HL, Lenz HJ, Marshall J, et al. Combination chemotherapy and ALVAC-CEA/B7.1 vaccine in patients with metastatic colorectal cancer. Clin Cancer Res 2008;14:4843-9. [PubMed]
- Hörig H, Lee DS, Conkright W, et al. Phase I clinical trial of a recombinant canarypoxvirus (ALVAC) vaccine expressing human carcinoembryonic antigen and the B7.1 co-stimulatory molecule. Cancer Immunol Immunother 2000;49:504-14. [PubMed]
- Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013;39:38-48. [PubMed]
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411-22. [PubMed]
- Rosenblatt J, Avivi I, Vasir B, et al. Vaccination with dendritic cell/tumor fusions following autologous stem cell transplant induces immunologic and clinical responses in multiple myeloma patients. Clin Cancer Res 2013;19:3640-8. [PubMed]
- Phuphanich S, Wheeler CJ, Rudnick JD, et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother 2013;62:125-35. [PubMed]
- Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med 1998;4:328-32. [PubMed]
- Gong J, Chen D, Kashiwaba M, et al. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med 1997;3:558-61. [PubMed]
- Lesterhuis WJ, de Vries IJ, Schuurhuis DH, et al. Vaccination of colorectal cancer patients with CEA-loaded dendritic cells: antigen-specific T cell responses in DTH skin tests. Ann Oncol 2006;17:974-80. [PubMed]
- Morse MA, Niedzwiecki D, Marshall JL, et al. A randomized phase II study of immunization with dendritic cells modified with poxvectors encoding CEA and MUC1 compared with the same poxvectors plus GM-CSF for resected metastatic colorectal cancer. Ann Surg 2013;258:879-86. [PubMed]
- Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity 2013;39:49-60. [PubMed]
- Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 2002;298:850-4. [PubMed]
- Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 2012;12:269-81. [PubMed]
- Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011;17:4550-7. [PubMed]
- Parkhurst MR, Yang JC, Langan RC, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther 2011;19:620-6. [PubMed]
- Morgan RA, Yang JC, Kitano M, et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010;18:843-51. [PubMed]
- Chodon T, Comin-Anduix B, Chmielowski B, et al. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin Cancer Res 2014;20:2457-65. [PubMed]
- Robbins PF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011;29:917-24. [PubMed]
- Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509-18. [PubMed]
- Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725-33. [PubMed]
- Weiner LM, Murray JC, Shuptrine CW. Antibody-based immunotherapy of cancer. Cell 2012;148:1081-4. [PubMed]
- Bronte G, Cicero G, Cusenza S, et al. Monoclonal antibodies in gastrointestinal cancers. Expert Opin Biol Ther 2013;13:889-900. [PubMed]
- Jiang XR, Song A, Bergelson S, et al. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat Rev Drug Discov 2011;10:101-11. [PubMed]
- Vacchelli E, Aranda F, Eggermont A, et al. Trial Watch: Tumor-targeting monoclonal antibodies in cancer therapy. Oncoimmunology 2014;3:e27048. [PubMed]
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122-33. [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [PubMed]
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [PubMed]
- Chung KY, Gore I, Fong L, et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol 2010;28:3485-90. [PubMed]
- Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 Ligands, and Other Features of the Tumor Immune Microenvironment with Response to Anti-PD-1 Therapy. Clin Cancer Res 2014. [Epub ahead of print]. [PubMed]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [PubMed]
- Galluzzi L, Senovilla L, Zitvogel L, et al. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov 2012;11:215-33. [PubMed]
- Kroemer G, Zitvogel L. Abscopal but desirable: The contribution of immune responses to the efficacy of radiotherapy. Oncoimmunology 2012;1:407-8. [PubMed]
- Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013;105:256-65. [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [PubMed]


