Regorafenib-induced transverse myelopathy after stereotactic body radiation therapy
Case Report
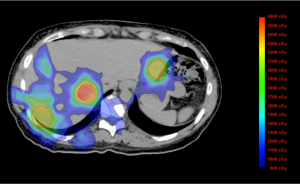
The patient is a 51-year-old woman whose screening colonoscopy in March 2010 demonstrated cecal cancer at the appendiceal orifice. Right hemi-colectomy removed a 3.5 cm moderately differentiated mucinous adenocarcinoma, positive for Kras G12V mutation, invading the appendix with 6 of 30 positive lymph nodes (pT4aN2aM0, Stage IIIC adenocarcinoma of the colon). Postoperatively, she received 12 cycles of mFOLFOX6 and remained without evidence of disease at the completion of treatment. In July 2011, the patient was found to have three discrete liver nodules on routine imaging that were highly suspicious for metastatic disease on a follow-up positron emission tomography-computed tomography (PET-CT). A 1.1 cm (anterior) lesion was located in segment 2; a 2.1 cm (lateral) lesion was in segment 6, and an 8 mm [inferior vena cava (IVC)] lesion in segment 7. The proximity of the segment 7 lesions to the IVC precluded surgery, and she was referred for stereotactic body radiation therapy (SBRT). The patient was immobilized using a full body vacuum cushion with abdominal compression and a 4-dimensional computed tomography (4D-CT) was acquired. After the gross tumor volume was delineated on 4D-CT, an internal treatment volume was developed based on tumor motion; an additional margin was placed to form the planning treatment volume. She was treated to 20 Gy in a single fraction to each of the three lesions over 11 days. For each individual plan, the mean and maximum spinal cord doses were 429.8 cGy and 849.7 cGy for the IVC lesion, 203.7 cGy and 367 cGy for the anterior lesion, 13.3 and 19.6 cGy for the lateral lesion. For the summation of all three plans, the mean and maximum spinal cord doses were 646.8 and 1,205 cGy (Figure 1). The patient developed nausea and abdominal discomfort that resolved with ibuprofen after the first treatment, but otherwise tolerated the treatment well.
Immediately after SBRT, she started treatment with capecitabine 2,000 mg daily given for 7 days every 2 weeks. After 9 months of treatment, persistent disease in the liver was identified via PET-CT and the patient underwent a right hepatectomy with partial vena caval resection. Subsequently, she received 12 cycles of FOLFIRI with zev-aflibercept. During the course of treatment, she noted shoulder pain radiating from her neck and paresthesiaes involving her forearms. Subsequently, in May 2013, she was found to have two lung nodules and two abdominal lymph nodes suspicious for metastatic disease, and was recommended treatment with regorafenib.
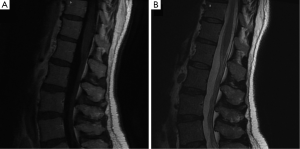
Within 2 weeks of starting regorafenib, the patient developed L’Hermitte’s sign with shooting pains radiating from her hips down the posterior aspect of both legs, resulting in an inability to bear weight and difficulty in ambulating. Neurological evaluation revealed diffuse hyperalgesia and dysesthesia of her lower extremities with hyperreflexia and a low thoracic sensory level, characteristic of transverse myelopathy. Magnetic resonance imaging (MRI) with contrast of the thoracic and lumbar spine revealed abnormal enhancement of the proximal cauda equina nerve roots, without evidence of metastatic disease, cord impingement or foraminal narrowing (Figure 2). A lumbar puncture was unremarkable, with acellular cytologically negative fluid and normal chemistries. Simultaneously, the patient developed grade 3 palmoplantar erythrodysesthesia manifesting as painful erythematous desquamation of her palms. Regorafenib was discontinued, and she was treated with dexamethasone, pentoxifylline, vitamin E, and emollients. At 2-week follow-up, she was recovering with lingering weakness, but no pain. She was neurologically normal 6 months later.
Discussion
Radiation myelopathy generally occurs at least 6 months after receiving radiation to the spinal cord, and has a diverse clinical presentation. It may manifest as sensory deficits, changes in gait, spasticity, weakness, paralysis, Brown-Séquard syndrome, autonomic dysfunction, or bowel/bladder incontinence. The underlying pathogenesis involves microvascular injury induced by ionizing radiation, leading to changes in local permeability in the white matter (1). This model has been supported by animal studies showing that white matter injury is preceded by loss of endothelial cells after receiving 25 Gy to the spinal cord (2).
SBRT delivers larger ablative doses of radiation over ≤5 fractions with a greater degree of accuracy that can be achieved with conventional techniques, but is not without limitations. Based on a model constructed from nine studies on myelopathy after SBRT, Kirkpatrick et al. determined the risk of myelopathy after receiving 20 Gy in three fractions to be less than 1% (3). Similarly, using dosimetric data from five cases, Sahgal et al. established a spinal cord tolerance threshold of 10 Gy in a single fraction, or a normalized biological effective dose (nBED) of 30-35 Gy2/2 up to five fractions (4). For the patient presented above, no single fraction delivered more than 8.5 Gy to the spinal cord, and no part of the cord received more than 1,205 cGy across all three treatments. Additionally, only a small fraction of the anatomically relevant spinal cord received the maximum dose, with the majority receiving between 900 and 1,200 cGy (Figure 1). Together, it is highly unlikely that radiation therapy alone accounts for the patient’s neurological symptoms.
SBRT has unique effects on the spinal cord not seen with convention fraction schemes. With SBRT, tumor killing occurs via microvasculature damage as a result of endothelial cell apoptosis and microvascular dysfunction, a phenomenon that is much less prominent with conventionally fractionated radiotherapy (5). Vascular endothelial growth factor (VEGF) is a key mediator of angiogenesis and tissue repair. Given this role, VEGF inhibition may compromise normal repair processes after radiation therapy, precipitating untoward effects in patients who would otherwise be asymptomatic.
Regorafenib is a receptor tyrosine kinase inhibitor indicated for metastatic colorectal cancer and locally advanced gastrointestinal stromal tumors (6). It has multiple targets, all implicated in various oncogenic phenomena: VEGF-R1/-R2/-R3 and TIE-2 in angiogenesis, KIT, RET, RAF1, BRAF in oncogenesis, FGFR-1 and PDGFR-α/β in the tumor microenvironment (6). Its inhibition of multiple overlapping pathways results in its effectiveness as anti-angiogenic therapy. Its most common adverse effects are fatigue, diarrhea, hypertension, and palmoplantar erythrodysesthesia (PPE) (7-9). PPE manifests as painful erythematous lesions occurring at sites of increased friction on the palms and soles, and has a 44× increased risk after regorafenib use compared to other tyrosine kinase inhibitors (10). Only two cases of severe sensory neuropathy were reported with regorafenib as a monotherapy, and each one case when patients were treated in combination with FOLFIRI or FOLFOX (11).
Radiation therapy and anti-angiogenesis compounds act synergistically for therapeutic advantage, but produces toxicity in the same manner. This synergistic effect is documented for gastrointestinal injury after SBRT and VEGF-targeted therapies (either bevacizumab, sorafenib, pazopanib, or sunitinib) (12). Hoang et al. found that bevacizumab alone inhibited endothelial cell growth and capillary formation (13). More importantly, bevacizumab given with radiation induced significantly higher levels of endothelial apoptosis after radiation, leading to reduced blood vessel formation. Increased rates of neurological toxicity have also been seen when radiation therapy is followed by bevacizumab. A case series by Kelly et al. included a patient with Brown-Séquard syndrome after receiving 30 Gy in 10 fractions to the spinal cord from T3-T5 (14). In this case, the adverse event was delayed. Anti-angiogenesis agents have also been used in a therapeutic capacity for radiation-induced central nervous system (CNS) toxicity, ostensibly by targeting VEGF to reduce blood-brain barrier permeability. In a randomized trial of 14 patients with cerebral radiation necrosis, bevacizumab use reduced the volume of cerebral necrosis and improved neurological function, suggesting that clinical outcomes after VEGF inhibition are tissue specific (15).
In summary, we hypothesize that off target effects of radiation treatment caused subclinical microvascular damage in the spinal cord, and the subsequent anti-angiogenic effects of regorafenib impaired neuronal repair. This is the first case to link regorafenib as a causal agent in precipitating myelopathy.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Schultheiss TE, Kun LE, Ang KK, et al. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys 1995;31:1093-112. [PubMed]
- Stewart PA, Vinters HV, Wong CS. Blood-spinal cord barrier function and morphometry after single doses of x-rays in rat spinal cord. Int J Radiat Oncol Biol Phys 1995;32:703-11. [PubMed]
- Kirkpatrick JP, van der Kogel AJ, Schultheiss TE. Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys 2010;76:S42-9. [PubMed]
- Sahgal A, Ma L, Gibbs I, et al. Spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2010;77:548-53. [PubMed]
- Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell 2005;8:89-91. [PubMed]
- Wilhelm SM, Dumas J, Adnane L, et al. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 2011;129:245-55. [PubMed]
- Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295-302. [PubMed]
- George S, Wang Q, Heinrich MC, et al. Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: a multicenter phase II trial. J Clin Oncol 2012;30:2401-7. [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [PubMed]
- Belum VR, Wu S, Lacouture ME. Risk of hand-foot skin reaction with the novel multikinase inhibitor regorafenib: a meta-analysis. Invest New Drugs 2013;31:1078-86. [PubMed]
- Schultheis B, Folprecht G, Kuhlmann J, et al. Regorafenib in combination with FOLFOX or FOLFIRI as first- or second-line treatment of colorectal cancer: results of a multicenter, phase Ib study. Ann Oncol 2013;24:1560-7. [PubMed]
- Barney BM, Markovic SN, Laack NN, et al. Increased bowel toxicity in patients treated with a vascular endothelial growth factor inhibitor (VEGFI) after stereotactic body radiation therapy (SBRT). Int J Radiat Oncol Biol Phys 2013;87:73-80. [PubMed]
- Hoang T, Huang S, Armstrong E, et al. Enhancement of radiation response with bevacizumab. J Exp Clin Cancer Res 2012;31:37. [PubMed]
- Kelly PJ, Dinkin MJ, Drappatz J, et al. Unexpected late radiation neurotoxicity following bevacizumab use: a case series. J Neurooncol 2011;102:485-90. [PubMed]
- Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys 2011;79:1487-95. [PubMed]


