
DNA damage repair deficiency as a predictive biomarker for FOLFIRINOX efficacy in metastatic pancreatic cancer
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the deadliest major cancer, with a 5-year survival rate of approximately 9%. The disease is projected to become the second leading cause of cancer death by the year 2030 (1). Most patients present with advanced, unresectable disease, and almost all patients who undergo curative intent surgery ultimately develop disease recurrence (2). Gemcitabine/nab-paclitaxel and FOLFIRINOX are the two first line regimens most commonly used for the treatment of metastatic PDAC. FOLFIRINOX is a four-drug regimen that includes folinic acid (leucovorin), 5-fluorouracil, irinotecan, and oxaliplatin which is a platinum drug. The choice of regimen is often made based on patient characteristics such as performance status, comorbidities, patient preference as well as the individual toxicity profiles of each regimen. The median overall survival (OS) among patient with metastatic PDAC treated with gemcitabine/nab-paclitaxel is only approximately 8.5 months (3,4). FOLFIRINOX is also a standard first line treatment option that has shown improvement in OS of up to 14 months (5,6). However, there are well known toxicities associated with the FOLFIRINOX regimen, including neutropenia, thrombocytopenia, sensory neuropathy, nausea, vomiting, diarrhea and fatigue, and criteria for patient selection is an unmet need (1,2). There are several molecular subtypes of PDAC (7). However, this classification is not yet useful to guide clinical decision-making.
Homologous repair deficiency (HRD) may define a subset of patients with PDAC. Patients with pathogenic variants in DNA damage repair (DDR) genes may derive greater benefit with platinum-based chemotherapy in metastatic PDAC (8,9). Platinum compounds such as oxaliplatin in FOLFIRINOX form inter- and intra-strand crosslinks that cause cell death via apoptosis. Compared to the other chemotherapeutic regimens as well as the other components of FOLFIRINOX, which inhibit DNA replication, the cross links and DNA breaks caused by oxaliplatin make the cell particularly vulnerable to deficiencies in DDR (10,11). DDR pathways, in particular nucleotide excision repair and homologous recombination, are central to the cellular response to platinum-based chemotherapy (10,12,13). These pathways are the main mechanism of platinum chemo-resistance (9), and can be used to predict response to platinum-based chemotherapy (2,14,15). In breast and ovarian cancer for example the presence of BRCA 1/2 pathogenic variants seem to predict response to platinum therapy (16-18). PDAC patients with pathogenic germline and somatic variants in BRCA and other DDR genes have shown improved OS in response to platinum agents (8,9,14,19). Estimates for prevalence of somatic pathogenic variants in DDR genes among PDAC patients range from 12–33% (14). Elucidation of tumor-specific pathogenic variants and molecular subtypes within this group could allow for targeted chemotherapeutic regimens to treat this highly metastatic and chemo-resistant disease. The aim of this study was to investigate the role of DDR genes as a predictive biomarker for response to first-line platinum chemotherapy with FOLFIRINOX in patients with metastatic PDAC.
Methods
This retrospective study included patients treated at the University of Miami Sylvester Comprehensive Cancer Center between 2012 and 2018. Patients were included if they had a confirmed diagnosis of metastatic PDAC and had germline and/or somatic genetic testing; only those who received FOLFIRINOX in the frontline setting were eligible. The University of Miami Institutional Review Board approved this analysis with a waiver for informed consent, as it was a retrospective, non-interventional study with some patients deceased at the time of the study.
The following demographic, clinical and pathologic variables were collected: sex, age, race/ethnicity, date of diagnosis, personal history of prior malignancies, family history of any malignancy, smoking status, diabetes, Eastern Cooperative Oncology Group (ECOG) performance status at diagnosis, baseline carbohydrate antigen 19-9 (CA19-9) level, location of pancreatic primary, location(s) of metastases, first line chemotherapy, second line chemotherapy, non-chemotherapy treatment history (surgery and/or radiation), start and end date of platinum chemotherapy, date of progression on platinum chemotherapy (defined as tumor enlargement or new metastases), and date of last follow up or date or death. Germline and somatic next generation sequencing results were collected from commercial testing laboratories. DDR genes considered to be relevant for this study are shown in Table 1.
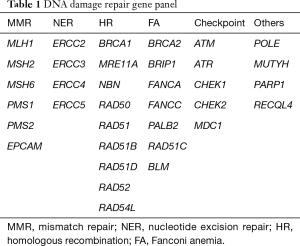
Full table
Kaplan-Meier analyses were used to estimate OS and progression free survival (PFS) for patients in this study. OS was defined as the time from the start date of frontline chemotherapy to date of death or last follow up and PFS was defined as the time from the start date of frontline chemotherapy to time of disease progression or death. Patients known to be alive were censored at the time of last contact. Statistical significance was defined as P<0.05, and all tests were two-sided. Tests were performed using the IBM SPSS statistics software version 22 (IBM, NY, USA). Descriptive statistics were used to summarize the baseline characteristics and pathogenic variants in DDR genes.
Results
Between 2012 and 2018, 116 patients with metastatic pancreatic adenocarcinoma underwent germline testing or somatic testing through next generation sequencing through several commercial platforms. Among these, 40 patients received chemotherapy with FOLFIRINOX in the first line setting. The median age was 59 years and 37.5% of patients were female. 70% of patients were Hispanic and 63% had de-novo metastatic disease and were thus treatment-naïve. Baseline characteristics are summarized in Table 2.
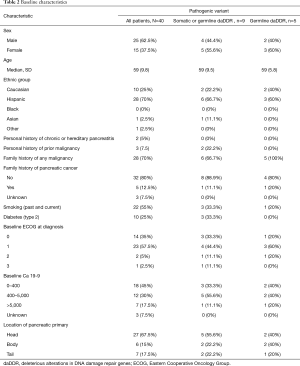
Full table
Germline testing revealed pathogenic variants in DDR genes in 5 patients in our cohort (12%), including 3 patients in BRCA2, one patient in RAD51C (20) and one in MUTYH (Table 3). Somatic next generation sequencing found an additional 4 patients (10%) with pathogenic variants in DDR genes including 2 with BRCA2 and one each with BRCA1 and POLE. The specific variants are summarized in Table 3. The median PFS was significantly longer in patients with any (germline or somatic) pathogenic variant in DDR genes than in those without alterations 18.5 vs. 6.9 months (log-rank P=0.003) as shown in Figure 1A. When restricted to the presence or absence of germline pathogenic variants in DDR genes, the median PFS was 18.5 vs. 7.4 months (log-rank P=0.005) as shown in Figure 1B.

Full table
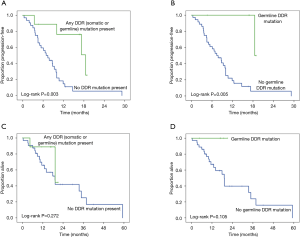
The median OS for the entire cohort was 11.5 months was not statistically different between the two groups. Nonetheless there were no deaths so far in the subgroup with germline pathogenic variants in DDR genes treated with frontline FOLFIRINOX (as shown in Figure 1).
Three patients with germline pathogenic variants in DDR genes (2 BRCA2 and 1 RAD51C) had complete or near-complete radiological and tumor marker responses to FOLFIRINOX and were treated with olaparib (a PARP inhibitor) as maintenance and remain progression-free after 16.8, 16.9 and 18.9 months respectively (the median PFS with FOLFIRINOX followed by olaparib in these patients was 16.9 months with all 3 patients still on treatment under follow-up).
Discussion
In this study, we have identified a subset of patients with metastatic PDAC and germline or somatic pathogenic variants in DDR genes, who have a statistically superior PFS when treated with the platinum-containing regimen FOLFIRINOX. There appears to be a trend towards superior OS as well in this subset of patients compared to patients who are wild-type for all DDR genes.
Within recent decades there has been an availability of new information as it regards to germline and sporadic pathogenic variants in the DDR gene pathways in pancreatic cancer. DNA repair serves two roles: survival of cancer cells from damage induced by genotoxic stress, and enabling cancer cells to accumulate genomic alterations that contribute to their aggressive phenotype (21). Furthermore, cancer DNA repair differs from normal cells such that most cancers will have lost at least one DDR pathway function during their generation, leading to a greater dependency on the remaining pathways (22).
A variety of germline pathogenic variants, including BRCA2, BRCA1, PALB2, CDKN2A and mismatch repair genes have been reported in pancreatic cancer with the highest prevalence being BRCA 1/2 estimated at about 5% (23). Indeed due to the high frequency of BRCA1/2 pathogenic variants in PDAC, NCCN criteria for BRCA1/2 germline testing have been recently updated to include testing for all patients with PDAC. Other pathogenic variants identified in this study include a RAD51C pathogenic variant, which is primarily associated with ovarian cancer risk, but which is also a known Fanconi anemia pathogenic variant, and which participates in HR. MUTYH, a base excision repair gene has not been associated with increased risk of pancreatic cancer either in monoallelic or in biallelic form and has not previously been reported (to our knowledge) in pancreatic cancer patients was also identified in this study. Pathogenic variants in these and other DDR genes lead to a deficiency in effective repair of DNA double-strand breaks via the mechanism of homologous recombination (18). The BRCA2 function has a more specific role in DNA repair, regulating the activity of RAD51, which is required for homologous recombination (24). Loss of function of these genes leads to HRD.
Platinum agents exert their cytotoxic effect by binding directly to DNA, causing crosslinking of DNA strands and thereby inducing DNA double strand breaks. This may make them more efficacious in the treatment of patients with DDR gene alterations, especially BRCA1/2 pathogenic variants, where the damage is not repaired effectively (25). Consequently, deleterious germline pathogenic variants can result in an exploitable DDR dependency, conferring a potential therapeutic target. This serves as a rationale for the findings seen in our study that a subset of patients with pancreatic cancer who harbor variants in DDR genes whether somatic or germline predicts sensitivity to platinum-based therapy. This study adds to the growing literature that has reported OS benefit in germline BRCA 1/2 carrier’s in those treated with platinum vs. non-platinum agents in the first line setting (19) and more recently a study who looked at a smaller cohort of patients treated with FOLFIRINOX with presence of DDR gene pathogenic variants vs. absence and demonstrated an improved OS in patients with presence of pathogenic variants (14).
The recently presented COMPASS trial studied 180 patients with pancreatic cancer underwent comprehensive molecular profiling with whole genome sequencing as well as RNA sequencing. They separated patients into two subgroups a Moffit (26) basal like signature and a classic signature. In the classical genotype patients seemed to benefit more from mFOLFIRINOX with a median OS that was 10.1 months in classical versus 6.6 moths in the basal-like subtypes (P=0.001) and a mPFS 7.1 vs. 2.6 months respectively. The HRD patients/duplicator phenotypes clustered in the classical genotype subgroup (27).
Additionally, our finding of three patients who remained progression-free after 16.8, 16.9, and 18.9 months respectively while on maintenance therapy with a PARP inhibitor mirrors recent advances in the literature such as the phase III POLO trial, which showed significant increases in PFS for metastatic platinum-sensitive PDAC patients with germline BRCA mutations who received maintenance therapy with a PARP inhibitor (9). Like platinum therapy, PARP inhibition impairs the repair of DNA breaks, and appears to be more effective in patients with homologous repair deficiencies such as with BRCA mutations (9,28).
In sum, with the advent of FOLFIRINOX in the first line setting, oxaliplatin has become the forefront platinum agent. Nonetheless given the toxicity associated with this regimen many patients are deemed ineligible. Therefore, defining biomarkers of platinum responsiveness would significantly alter our treatment of choice for an individual patient and this would hopefully in turn lead to an improvement in patient outcomes. This study supports DDR as a predictive biomarker for platinum response. There are several strengths to our study; we restricted inclusion to metastatic patients who all received FOLFIRINOX in the upfront setting. We also had access to all the patient outcomes data. Lastly, we looked at both germline and somatic DDR pathogenic variants and their relationship with PFS and OS.
There were inherent limitations in this study. It was a single-institution, non-randomized, retrospective study, which makes it difficult to formulate definitive conclusions regarding the best therapeutic options. Additionally, the choice of chemotherapy was dependent on the discretion of the treating oncologist. Furthermore, the interpretation of results is limited due to the small patient population and relatively short follow-up period. Although this study did not identify a statistically superior OS in patients with pathogenic DDR gene variants versus wild-type patients, it is likely that statistical significance could be demonstrated with more mature follow-up.
In conclusion, we present a small retrospective study identifying a PFS benefit among those patients with pathogenic variants in DDR genes who were treated with platinum-containing regimen, namely FOLFIRINOX. Moreover, we also demonstrated that the benefit was regardless of germline or somatic variant status. These findings are hypothesis generating and the role of DDR pathogenic variants as a predictive biomarker for FOLFIRINOX benefit should be further evaluated in prospective trials.
Acknowledgments
None.
Footnote
Conflicts of Interest: This was presented as a Poster presentation at the 2019 ASCO Gastrointestinal Cancers Symposium.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States (Cancer Research (2014) 74 (2913-21)). Cancer Res 2014;74:4006. [Crossref]
- Knudsen ES, O'Reilly EM, Brody JR, et al. Genetic Diversity of Pancreatic Ductal Adenocarcinoma and Opportunities for Precision Medicine. Gastroenterology 2016;150:48-63. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. New Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Goldstein D, El-Maraghi RH, Hammel P, et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic cancer: Long-term survival from a phase III trial. J Natl Cancer Inst 2015. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. New Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Pacheco-Barcia V, France T, Zogopoulos G, et al. P-164Gemcitabine plus nab-paclitaxel versus modified FOLFIRINOX as first line chemotherapy in metastatic pancreatic cancer: A comparison of toxicity and survival. Ann Oncol 2018. [Crossref]
- Bailey P, Chang DK, Nones K. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531:47-52. [Crossref] [PubMed]
- Pihlak R, Valle JW, McNamara MG. Germline mutations in pancreatic cancer and potential new therapeutic options. Oncotarget 2017;8:73240-57. [Crossref] [PubMed]
- Waddell N, Pajic M, Patch A, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015;518:495-501. [Crossref] [PubMed]
- Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med 2006;12:440-50. [Crossref] [PubMed]
- Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature 2012;481:287-94. [Crossref] [PubMed]
- Jung Y, Lippard SJ. Direct cellular responses to platinum-induced DNA damage. Chem Rev 2007;107:1387-407. [Crossref] [PubMed]
- Gavande NS, VanderVere-Carozza PS, Hinshaw HD, et al. DNA repair targeted therapy: The past or future of cancer treatment? Pharmacol Ther 2016;160:65-83. [Crossref] [PubMed]
- Sehdev A, Gbolahan O, Hancock BA, et al. Germline and somatic DNA damage repair gene mutations and overall survival in metastatic pancreatic adenocarcinoma patients treated with FOLFIRINOX. Clin Cancer Res 2018;24:6204-11. [Crossref] [PubMed]
- Sahin IH, Lowery MA, Stadler ZK, et al. Genomic instability in pancreatic adenocarcinoma: a new step towards precision medicine and novel therapeutic approaches. Expert Rev Gastroenterol Hepatol 2016;10:893-905. [PubMed]
- Lips EH, Mulder L, Oonk A, et al. Triple-negative breast cancer: BRCAness and concordance of clinical features with BRCA1-mutation carriers. Br J Cancer 2013;108:2172-7. [Crossref] [PubMed]
- McCabe N, Turner NC, Lord CJ, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res 2006;66:8109-15. [Crossref] [PubMed]
- Turner N, Tutt A, Ashworth A. Hallmarks of 'BRCAness' in sporadic cancers. Nat Rev Cancer 2004;4:814-9. [Crossref] [PubMed]
- Golan T, Sella T, O'Reilly EM, et al. Overall survival and clinical characteristics of BRCA mutation carriers with stage I/II pancreatic cancer. Br J Cancer 2017;116:697-702. [Crossref] [PubMed]
- Palacio S, Pollack T, Silva-Smith R, et al. Exceptional response to FOLFIRINOX in a patient with pancreatic cancer and a germline RAD51C mutation. J Gastrointest Oncol 2018;9:E19-22. [Crossref] [PubMed]
- Dietlein F, Thelen L, Reinhardt HC. Cancer-specific defects in DNA repair pathways as targets for personalized therapeutic approaches. Trends Genet 2014;30:326-39. [Crossref] [PubMed]
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature 2009;461:1071-8. [Crossref] [PubMed]
- Holter S, Borgida A, Dodd A, et al. Germline BRCA mutations in a large clinic-based cohort of patients with pancreatic adenocarcinoma. J Clin Oncol 2015;33:3124-9. [Crossref] [PubMed]
- Tutt A, Ashworth A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol Med 2002;8:571-6. [Crossref] [PubMed]
- Lowery MA, Kelsen DP, Stadler ZK, et al. An emerging entity: Pancreatic adenocarcinoma associated with a known brca mutation: Clinical descriptors, treatment implications, and future directions. Oncologist 2011;16:1397-402. [Crossref] [PubMed]
- Moffitt RA, Marayati R, Flate EL, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet 2015;47:1168-78. [Crossref] [PubMed]
- O'Kane GM, Fischer S, Denroche R, et al. Integrative molecular profiling and response to chemotherapy on the COMPASS trial. JCO 2019;37:188. [Crossref]
- Sharbeen G, McCarroll J, Goldstein D, et al. Exploiting base excision repair to improve therapeutic approaches for pancreatic cancer. Front Nutr 2015;2:10. [Crossref] [PubMed]


