
Preoperative elevated plasma fibrinogen level predicts tumor recurrence and poor prognosis in patients with hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer death worldwide, and China alone accounts for about half of total cases (1). The high prevalence of chronic hepatitis B virus (HBV) infection and HBV-related cirrhosis are the major risk factors for HCC development in China (2). Due to the asymptomatic feature of early HCC, many patients are found with advanced stages when diagnosed and lose their opportunities to get radical treatments, including liver transplantation, surgical resection and ablation (3). With the development of diagnostic techniques and extensive surveillance programs for populations with high risk, the proportion of patients diagnosed with early stage and receiving curative therapies are increasing, but the long-term prognosis of these patients are still poor, especially with high rate of recurrence (4). Therefore, it’s necessary to look for some novel biomarkers to recognize and stratify patients with high risk of recurrence and allocate individual follow-up algorithms and preventive measures to improve the clinical outcomes.
A specific relationship between blood coagulation system and cancer has been revealed for decades. Tumor cells may possess procoagulant activities and could facilitate local activation of coagulation system, which may promote the tumor progression, angiogenesis and hematogenous metastasis (5-8). Fibrinogen is a kind of plasma glycoprotein synthesized by hepatocytes, which acts as an important coagulation factor and can be converted into fibrin by thrombin (9). And fibrinogen also involves in inflammatory reaction in response to injury, infection or inflammation (10). Previous clinical studies have revealed that the levels of pretreatment plasma fibrinogen are elevated and are associated with tumor progression and poor prognosis in several malignant tumors (11-17). As for HCC, Zhu et al. reported that the mRNA expression levels of fibrinogen gamma were up-regulated both in HCC cell lines and tissues, and elevated plasma fibrinogen levels were correlated with the presence of tumor thrombosis (18). Then, Kinoshita et al. found that elevated plasma fibrinogen levels were independently associated with poor prognosis in HCC patients (19). And several following studies have focused on the fibrinogen as a prognostic predictor for overall survival (OS) and tumor recurrence in HCC patients (20-22). But the evidences are limited, and the patients enrolled in these studies usually with an early Barcelona Clinic Liver Cancer (BCLC) stage HCC. Besides, our center first investigated the prognostic value of fibrinogen in HCC patients receiving liver transplantation and demonstrated it as a new predictor of HCC recurrence (23). The present study aimed to investigate the association between plasma fibrinogen and clinicopathological characteristics and clarify the prognostic value of plasma fibrinogen in tumor recurrence and OS in HCC patients with a wider range of BCLC stages after surgical resection.
Methods
Patients
A total of 466 patients with primary liver cancer from March 2005 to May 2013 were retrospectively collected. The eligibility criteria are as follows: (I) histologically diagnosed with HCC; (II) no extrahepatic or distant metastases; (III) received liver resection without pre-/intra-operative treatment; (IV) no other concomitant malignances or hematological diseases; (V) age equal to or greater than 18 years old; (VI) complete clinical and laboratory data were available; (VII) followed up adequately. According to the eligibility rules, 302 HCC patients were included and analyzed in our study, while 164 cases were excluded: 57 cases received preoperative treatment, 73 cases undergone ablation treatment due to multiple tumor nodules, 13 cases were histologically proven to be not HCC, 20 cases were found presence with tumor rupture, and one case at seventeen years old was also excluded. Before surgery, all patients were examined by an enhanced computed tomography (CT) or magnetic resonance imaging (MRI) and clinically diagnosed with HCC. When these images were reviewed, tumor-related parameters, such as tumor diameter, the number of tumor lesions and vascular invasion, were evaluated and collected respectively.
Preoperative plasma fibrinogen and other variables
Blood samples were obtained within 7 days before surgery and tested in the clinical laboratory of our hospital. Plasma fibrinogen levels were measured by the Clauss method with fibrinogen reagent kit (Diagnostica Stago, Asnières sur Seine, France) and the normal range was defined at the levels between 2.0 and 4.0 g/L. Besides, other indexes including neutrophil, lymphocyte and platelet (PLT) count, alanine aminotransferase (ALT), albumin (ALB), glutamyl transpeptidase (GGT), α-fetoprotein (AFP) and HBV-DNA were also collected. Neutrophil-lymphocyte ratio (NLR) was calculated by neutrophil and lymphocyte count. The Model for End-stage Liver Disease (MELD) score was calculated and rounded to an integer value by the equation with 3.8 × ln[bilirubin (mg/dL)] + 11.2 × ln(INR) + 9.6 × ln[creatinine (mg/dL)] + 6.4. The presence of liver cirrhosis was identified histologically with specimen obtained in operation. The Child-Pugh grade and BCLC stage were also evaluated respectively.
Follow-up
All patients were followed up regularly at the outpatient office after liver resection. The serum AFP levels were measured every month in the first year and then every three months for the next two years. Abdominal ultrasound and dynamic enhanced CT or MRI were performed every three months in the first two years and followed by once every six months in the third year. The follow-up programme began at the date of operation and ended with death or time of the last follow-up encompassed by this study (December 2016). At the end of follow-up period, patients didn’t occur tumor recurrence or HCC-related death were considered as failure events for DFS and OS respectively, while patients lost to follow up or died due to other non-HCC related deaths during follow-up period were considered as censored events.
Statistical analysis
Continuous variables were presented as median and ranges. Categorical variables were presented as numbers and percentages. The cut-off values of NLR and MELD score were determined by the receiver operating characteristic (ROC) curve with Youden’s index, while the cut-off value of fibrinogen was determined by the upper reference limit which recommended by the fibrinogen reagent kit, which was mainly in consideration of clinical easy application (14). The Pearson chi-square (χ2) test or Fisher’s exact test was used to analyze the association between plasma fibrinogen levels and other clinicopathological factors.
The disease-free survival (DFS) and OS rates were calculated by Kaplan-Meier method and compared by the log-rank test. Univariate Cox analysis was performed to identify potential related factors for HCC recurrence or OS. In consideration of other confounding factors and the impact of a suppressor effect, variables with a significant value of P<0.1 were subjected to a multivariate Cox proportional hazard analysis and further screened by forward selection method to evaluate their independent effect. All the variables selected for multivariate COX analyses were satisfied with the proportional hazard assumptions (data not shown). Hazard ratio (HR) and 95% confidence interval (CI) were also calculated for each other. Besides, nomograms based on the results of multivariate analysis were constructed to estimate the 3-, 5-year DFS and OS of HCC patients. The prediction performance was evaluated by a concordance index (C-index) and showed with a calibration plot.
All P values resulted from two-sided statistical testing. And a P value <0.05 was considered statistically significant. All data analyses were performed using the SPSS software package version 19.0 (SPSS, Chicago, IL, USA) and R version 3.2.3 with the package of “rms”.
Results
Patient characteristics
The baseline characteristics of enrolled 302 HCC patients are listed in Table 1. In this study, 266 (88.1%) patients were males and 36 (11.9%) were females. The median age of these patients was 51 years (range, 42.0–59.0 years). All patients were found with positive hepatitis B surface antigen, and six (2.0%) patients were present with antibodies to hepatitis C virus concurrently, while only one (0.3%) patient was diagnosed with coinfection of HIV. Two hundred and four (66.7%) patients were confirmed with liver cirrhosis by pathology. Besides, 58 (19.2%) and 96 (31.8%) patients had the history of alcohol consumption and smoking, respectively. All the patients, with well-preserved liver function at Child-Pugh grade A (92.7%) and B (7.3%), received surgical resection as their first treatment. And the median MELD score for overall HCC patients was 5 (range, 3.0–7.0). According to the Barcelona Clinic Liver Cancer (BCLC) system, 154 (51%) patients were classified with very early (BCLC 0) and early-stage (BCLC A) HCC, while 122 (40.4%) and 26 (8.6%) patients belonged to intermediate (BCLC B) and advanced-stage (BCLC C) HCC respectively. During the follow-up period (median =24.2 months), 193 (63.9%) patients developed tumor recurrence and 51 (16.9%) patients died.
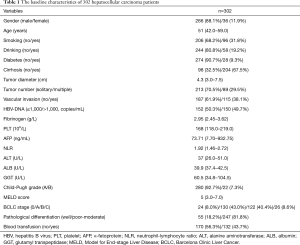
Full table
Comparison of preoperative plasma fibrinogen levels and clinicopathological characteristics
The median value of preoperative plasma fibrinogen level was 2.95 g/L. Patients were divided into two groups (≤4 g/L, n=258; >4 g/L, n=44) based on the upper limit of the normal range of plasma fibrinogen level. The associations of preoperative plasma fibrinogen levels with clinicopathological characteristics are presented in Table 2. The findings showed that the elevated plasma fibrinogen levels were associated with larger tumor size (P<0.001), higher NLR level (P=0.005), lower MELD score (P=0.001), the presence of vascular invasion (P<0.001), advanced BCLC stage (P<0.001) and poor-moderate pathological differentiation (P=0.020). No other clinicopathological parameters were found associated with plasma fibrinogen levels, such as gender, age, smoking, drinking, diabetes, cirrhosis, tumor number, HBV-DNA, AFP or Child-Pugh grade (all P>0.05).
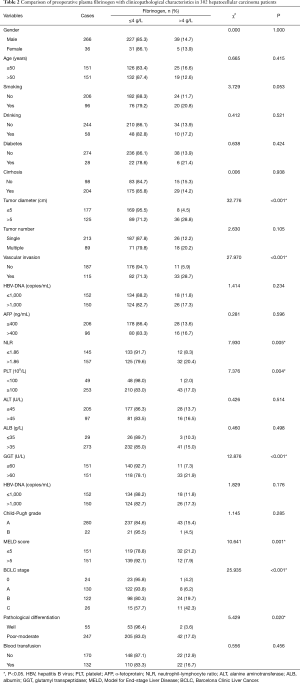
Full table
Prognostic factors of DFS in HCC patients
The 1-, 3-, 5-year DFS and OS rates were 56.6%, 32.0%, 25.9%. To identify the prognostic factors of DFS, both univariate and multivariate analyses were used to evaluate the effect of preoperative plasma fibrinogen and other clinicopathological variables. The results suggested that preoperative plasma fibrinogen (P<0.001), tumor diameter (P<0.001), tumor number (P<0.001), vascular invasion (P<0.001), AFP (P=0.027), NLR (P=0.008), GGT (P=0.001), smoking (P=0.037), BCLC stage (P<0.001), tumor differentiation (P=0.001) and blood transfusion (P=0.001) were related to DFS. The multivariate analysis showed that preoperative plasma fibrinogen (P=0.011), tumor diameter (P=0.001), tumor number (P<0.001), and blood transfusion (P=0.003) were independent factors to DFS (shown in Table 3).
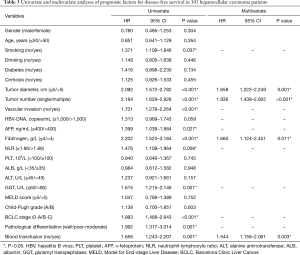
Full table
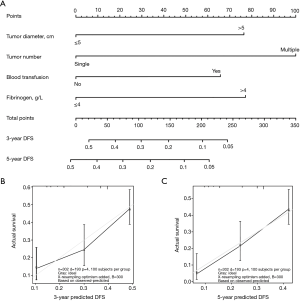
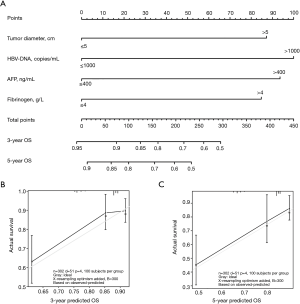
Furthermore, we constructed a nomogram for DFS prediction with four variables significant in multivariate analysis (Figure 1). The model displays a C-index of 0.656 (95% CI: 0.588–0.724).
Prognostic factors of OS in HCC patients
The 1-, 3-, 5-year OS rates were 93.8%, 81.5%, 71.8%. The results of univariate analysis showed that the preoperative plasma fibrinogen (P<0.001), tumor diameter (P<0.001), vascular invasion (P=0.001), AFP (P=0.002), GGT (P=0.009), HBV-DNA (P=0.002), age (P=0.022) and BCLC stage (P=0.002) were associated with OS. Furthermore, in multivariate analysis, preoperative plasma fibrinogen (P=0.025), tumor diameter (P=0.017), AFP (P=0.006) and HBV-DNA (P=0.005) were found to be independent prognostic factors of OS (shown in Table 4).
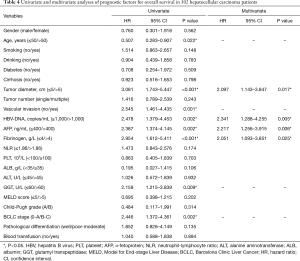
Full table
The nomogram was developed to predict the OS with above four variables significant in multivariate analysis (Figure 2). And the C-index of this model is 0.720 (95% CI: 0.637–0.803).
Prognostic values of preoperative plasma fibrinogen in HCC patients
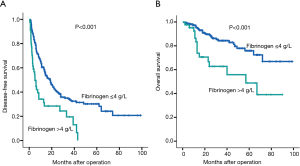
The 1-, 3-, 5-year DFS rates in patients with elevated plasma fibrinogen levels >4 g/L were 34.2%, 19.5% and 0.0%, respectively. These survival rates were significantly lower than the rates in patients with normal plasma fibrinogen level ≤4 g/L, which were 60.4%, 34.2% and 30.2%, respectively (P<0.001; Figure 3A). Similarly, the 1-, 3-, 5-year OS rates were also significantly lower in patients with elevated plasma fibrinogen levels >4 g/L than in patients with plasma fibrinogen levels ≤4 g/L (83.4%, 62.7% and 48.8% vs. 95.4%, 84.3% and 75.8%, respectively, P<0.001; Figure 3B).
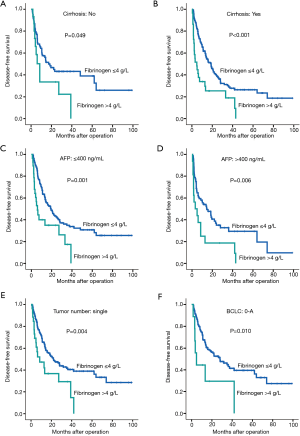
Furthermore, relevant subgroup analyses were also performed to analyze the prognostic values of preoperative plasma fibrinogen levels in specific HCC patients. We found that preoperative plasma fibrinogen was a prognostic factor of DFS (P=0.049, Figure 4A) in patients without cirrhosis. And as expected, similar results were also found in patients with cirrhosis (P<0.001, Figure 4B). Besides, in patients with different levels of AFP (≤400, >400 ng/mL), elevated plasma fibrinogen still showed as a prognostic factor with lower DFS rates (P=0.001, and P=0.006; Figure 4C,D). Both in patients with single tumor and BCLC 0-A stage, the elevated plasma fibrinogen levels also presented significantly prognostic values with lower DFS rates (P=0.004, Figure 4E; BCLC 0-A stage: P=0.010, Figure 4F).
Discussion
In the present study, we examined the relationships between preoperative plasma fibrinogen and clinicopathological characteristics and its prognostic significance in HCC patients. We found that an elevated plasma fibrinogen level was positively associated with larger tumor size, the presence of vascular invasion, advanced BCLC stage, lower MELD score, poorer pathological differentiation, and higher NLR. Moreover, our results demonstrated that patients with elevated preoperative plasma fibrinogen levels possessed worse OS and DFS. And in subgroup analyses, we found that elevated fibrinogen levels also related to poorer DFS in patients without cirrhosis, single tumor nodule, low AFP level and early stages, respectively. Our findings indicate that preoperative plasma fibrinogen level might reflect the tumor burden and progression, and act as a significantly independent prognostic marker in HCC.
Hyperfibrinogenemia has been recognized in many solid malignancies as a prognostic indicator with tumor progression and poor survival outcomes (11-17). And results from a meta-analysis also supported that pretreatment plasma fibrinogen represents as a biomarker of worse survival in tumor patients (24). Zhu et al. first reported elevated plasma fibrinogen levels correlating with advanced tumor stages and the presence of tumor thrombosis in HCC patients (18), which is consistent with our study. Moreover, recent clinical studies have also investigated the prognostic role of plasma fibrinogen in HCC patients (19-23). And in the current study, we further evaluated the prognostic value of preoperative plasma fibrinogen in several specific subgroups of HCC patients, such as patients without cirrhosis, with low AFP level, single tumor nodule, and early BCLC stages, and the results showed similar significant prognostic value as in the whole cohort, which indicated the universally applicable prognostic feature of fibrinogen.
Fibrinogen is a soluble 340-kDa glycoprotein synthesized by hepatocytes. A growing body of evidence has shown that fibrinogen participates in tumor development at different stages (25). Fibrinogen could bind directly to some growth factors, such as transforming growth factor-B (TGF-B), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and platelet-derived growth factor (PDGF) families, and contact with tumor cells to regulate tumor cell proliferation, inhibition of apoptosis, angiogenesis, and metastasis (26,27). Besides, as a prominent component in the coagulation pathway, fibrinogen could be converted to fibrin by the activated thrombin and facilitate platelet aggression by conjunction with platelets. It has been recognized that high levels of fibrinogen receptors expressed in some tumor cells, including intercellular adhesion molecule-1 (ICAM-1) and the α5β1 and αvβ3 integrins (28,29). Thereby fibrinogen promotes the contact between platelets and circulating tumor cells (CTCs) as a bridge, helping platelets adhesion to tumor cells (30). With the formation of such matrix barrier, the CTCs escape from the natural killer-cell induced elimination (31). Fibrinogen could also mediate the endothelial adhesion of CTCs by ICAM-1 on endothelial cells (32). Thus, fibrinogen may take an important part in the adhesive interaction among tumor cells, platelets, or endothelial cells, leading to hematogenous metastasis (33,34). On the other hand, Palumbo et al. demonstrated that spontaneous hematogenous and lymphatic metastasis was diminished in fibrinogen-deficient mice and indicated a therapeutic strategy focusing on hemostatic factors in controlling solid tumor metastasis (33). A recent study has shown that some anticoagulants possess the antitumor and antimetastatic properties both in vivo and in vitro (35). Furthermore, several studies have found that fibrinogen could be synthesized by tumor cells endogenously and induce the epithelial-to-mesenchymal transition (EMT), which involves in tumor cell migration, invasion, and metastasis (11,36).
In addition, fibrinogen also acts as an acute phase protein in response to infection or systemic inflammation (10). Previous studies have demonstrated that fibrinogen could regulate the inflammatory response by producing several pro-inflammatory cytokines (IL-1b, IL-6, and TNF-a) or inducing the interaction between leukocyte and endothelial cells (34,37). And the fibrinogen-modulated inflammatory response has been identified with cancer progression in tumor microenvironment (38). In our study, we also found that elevated plasma fibrinogen level is correlated with higher NLR, a noteworthy marker of inflammatory response, which is in accordance with previous studies (22,39). And Fu et al. have shown an enhanced prognostic value by combining preoperative fibrinogen and NLR in patients with HCC after liver transportation (22).
With various evidence from experimental and clinical studies, fibrinogen shows the potential to become a promising predictor for long-term prognosis of patients with malignant tumors. And some researchers have indicated the possibility of fibrinogen to be applied in cancer staging and patient stratification, aiming to improve personalized treatment selection, recurrence prevention and survival extension. Besides, plasma fibrinogen level is routinely tested before operation in clinical practice, which makes it available to be widely used.
There are several potential limitations in our study. First, as a retrospective study, all patients are enrolled from our single center and data missing is inevitable, and a limited sample size may cause an inappropriate conclusion. Second, except for fibrinogen, other inflammatory indicators such as C-reactive protein, were not included and analyzed, which may result in some statistical biases. Third, the plasma fibrinogen level after surgery and the expression level in tumor weren’t tested or collected. However, such work may be our following plan, and large-scale multi-center prospective validation study is further required.
Conclusions
In conclusion, our study has demonstrated that preoperative elevated plasma fibrinogen levels were associated with larger tumor size, the presence of vascular invasion, and advanced BCLC stages in patients with HCC. And it could serve as a significant useful prognostic predictor in HCC patients with poor long-term OS and DFS.
Acknowledgments
Funding: This work was supported by the Guangdong Natural Science Foundation (2016A030313278, 2015A030313038, 2015A030312013); Science and Technology Program of Guangzhou City (2014Y2-00200, 201604020001, 201508020262, 201400000001-3, 201607010024); Science and Technology Program of Guangdong Province (2017B020209004, 20169013); National 13th Five-Year Science and Technology Plan Major Projects of China (2017ZX10203205-006-001); and Guangdong Key Laboratory of Liver Disease Research (2017B030314027).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of the Third Affiliated Hospital of Sun Yat-sen University and written informed consent was obtained from all patients.
References
- Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2016;2:16018. [Crossref] [PubMed]
- Hemming AW, Berumen J, Mekeel K. Hepatitis B and Hepatocellular Carcinoma. Clin Liver Dis 2016;20:703-20. [Crossref] [PubMed]
- Tang H, Huang Y, Duan W, et al. A concise review of current guidelines for the clinical management of hepatocellular carcinoma in Asia. Transl Cancer Res 2017;6:1214-25. [Crossref]
- Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016;150:835-53. [Crossref] [PubMed]
- Rickles FR, Edwards RL. Activation of blood coagulation in cancer: Trousseau's syndrome revisited. Blood 1983;62:14-31. [Crossref] [PubMed]
- Im JH, Fu W, Wang H, et al. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer Res 2004;64:8613-9. [Crossref] [PubMed]
- Zacharski LR, Wojtukiewicz MZ, Costantini V, et al. Pathways of coagulation/fibrinolysis activation in malignancy. Semin Thromb Hemost 1992;18:104-16. [Crossref] [PubMed]
- Wojtukiewicz MZ, Zacharski LR, Moritz TE, et al. Prognostic significance of blood coagulation tests in carcinoma of the lung and colon. Blood Coagul Fibrinolysis 1992;3:429-37. [Crossref] [PubMed]
- Tennent GA, Brennan SO, Stangou AJ, et al. Human plasma fibrinogen is synthesized in the liver. Blood 2007;109:1971-4. [Crossref] [PubMed]
- Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol 2012;34:43-62. [Crossref] [PubMed]
- Shu YJ, Weng H, Bao RF, et al. Clinical and prognostic significance of preoperative plasma hyperfibrinogenemia in gallbladder cancer patients following surgical resection: a retrospective and in vitro study. BMC Cancer 2014;14:566. [Crossref] [PubMed]
- Pichler M, Hutterer GC, Stojakovic T, et al. High plasma fibrinogen level represents an independent negative prognostic factor regarding cancer-specific, metastasis-free, as well as overall survival in a European cohort of non-metastatic renal cell carcinoma patients. Br J Cancer 2013;109:1123-9. [Crossref] [PubMed]
- Qi Q, Geng Y, Sun M, et al. Hyperfibrinogen Is Associated With the Systemic Inflammatory Response and Predicts Poor Prognosis in Advanced Pancreatic Cancer. Pancreas 2015;44:977-82. [Crossref] [PubMed]
- Li H, Zhao T, Ji X, et al. Hyperfibrinogenemia predicts poor prognosis in patients with advanced biliary tract cancer. Tumour Biol 2016;37:3535-42. [Crossref] [PubMed]
- Zeng Q, Xue N, Dai D, et al. A Nomogram based on Inflammatory Factors C-Reactive Protein and Fibrinogen to Predict the Prognostic Value in Patients with Resected Non-Small Cell Lung Cancer. J Cancer 2017;8:744-53. [Crossref] [PubMed]
- Mei Y, Liu H, Sun X, et al. Plasma fibrinogen level may be a possible marker for the clinical response and prognosis of patients with breast cancer receiving neoadjuvant chemotherapy. Tumour Biol 2017;39:1010428317700002. [Crossref] [PubMed]
- Yu X, Hu F, Yao Q, et al. Serum fibrinogen levels are positively correlated with advanced tumor stage and poor survival in patients with gastric cancer undergoing gastrectomy: a large cohort retrospective study. BMC Cancer 2016;16:480. [Crossref] [PubMed]
- Zhu WL, Fan BL, Liu DL, et al. Abnormal expression of fibrinogen gamma (FGG) and plasma level of fibrinogen in patients with hepatocellular carcinoma. Anticancer Res 2009;29:2531-4. [PubMed]
- Kinoshita A, Onoda H, Imai N, et al. Elevated plasma fibrinogen levels are associated with a poor prognosis in patients with hepatocellular carcinoma. Oncology 2013;85:269-77. [Crossref] [PubMed]
- Zhang X, Long Q. Elevated serum plasma fibrinogen is associated with advanced tumor stage and poor survival in hepatocellular carcinoma patients. Medicine 2017;96:e6694. [Crossref] [PubMed]
- Liu Z, Guo H, Gao F, et al. Fibrinogen and D-dimer levels elevate in advanced hepatocellular carcinoma: High pretreatment fibrinogen levels predict poor outcomes. Hepatol Res 2017;47:1108-17. [Crossref] [PubMed]
- Fu SJ, Ji F, Han M, et al. Prognostic value of combined preoperative fibrinogen and neutrophil-lymphocyte ratio in patients with hepatocellular carcinoma after liver transplantation. Oncotarget 2017;8:4301-12. [PubMed]
- Wang GY, Jiang N, Yi HM, et al. Pretransplant Elevated Plasma Fibrinogen Level is a Novel Prognostic Predictor for Hepatocellular Carcinoma Recurrence and Patient Survival Following Liver Transplantation. Ann Transplant 2016;21:125-30. [Crossref] [PubMed]
- Perisanidis C, Psyrri A, Cohen EE, et al. Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: A systematic review and meta-analysis. Cancer Treat Rev 2015;41:960-70. [Crossref] [PubMed]
- Polterauer S, Grimm C, Seebacher V, et al. Plasma Fibrinogen Levels and Prognosis in Patients with Ovarian Cancer: A Multicenter Study. Oncologist 2009;14:979-85. [Crossref] [PubMed]
- Martino MM, Briquez PS, Ranga A, et al. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci U S A 2013;110:4563-8. [Crossref] [PubMed]
- Witsch E, Sela M, Yarden Y. Roles for Growth Factors in Cancer Progression. Physiology 2010;25:85-101. [Crossref] [PubMed]
- Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 2010;10:9-22. [Crossref] [PubMed]
- Duperray A, Languino LR, Plescia J, et al. Molecular identification of a novel fibrinogen binding site on the first domain of ICAM-1 regulating leukocyte-endothelium bridging. J Biol Chem 1997;272:435-41. [Crossref] [PubMed]
- Zheng S, Shen J, Jiao Y, et al. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci 2009;100:859-65. [Crossref] [PubMed]
- Palumbo JS, Talmage KE, Massari JV, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 2005;105:178-85. [Crossref] [PubMed]
- Roche Y, Pasquier D, Rambeaud JJ, et al. Fibrinogen mediates bladder cancer cell migration in an ICAM-1-dependent pathway. Thromb Haemost 2003;89:1089-97. [Crossref] [PubMed]
- Palumbo JS, Kombrinck KW, Drew AF, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood 2000;96:3302-9. [Crossref] [PubMed]
- Languino LR, Plescia J, Duperray A, et al. Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1-dependent pathway. Cell 1993;73:1423-34. [Crossref] [PubMed]
- Bobek V. Anticoagulant and fibrinolytic drugs - possible agents in treatment of lung cancer? Anticancer Agents Med Chem 2012;12:580-8. [Crossref] [PubMed]
- Sahni A, Simpson-Haidaris PJ, Sahni SK, et al. Fibrinogen synthesized by cancer cells augments the proliferative effect of fibroblast growth factor-2 (FGF-2). J Thromb Haemost 2008;6:176-83. [Crossref] [PubMed]
- Jennewein C, Tran N, Paulus P, et al. Novel aspects of fibrin(ogen) fragments during inflammation. Mol Med 2011;17:568-73. [Crossref] [PubMed]
- Steinbrecher KA, Horowitz NA, Blevins EA, et al. Colitis-Associated Cancer Is Dependent on the Interplay between the Hemostatic and Inflammatory Systems and Supported by Integrin M 2 Engagement of Fibrinogen. Cancer Res 2010;70:2634-43. [Crossref] [PubMed]
- Arigami T, Okumura H, Matsumoto M, et al. Analysis of the Fibrinogen and Neutrophil Lymphocyte Ratio in Esophageal Squamous Cell Carcinoma. Medicine 2015;94:e1702. [Crossref] [PubMed]


