Is hepatic resection better than transarterial chemoembolization in hepatocellular carcinoma with portal vein tumor thrombosis?
Introduction
As the fifth most common type of cancer and third most common cause of cancer-related mortality, hepatocellular carcinoma (HCC) continues to remain a public health concern worldwide. More than 800,000 people are diagnosed with HCC each year and the most common age of presentation is between 30–50 years old (1). If left untreated, the 5-year survival for HCC is less than 12% (2). The majority of HCC cases are caused on a background of chronic liver disease induced by inflammation due to viruses (HBV and HCV), toxins (especially alcohol), or metabolic disease. In developed nations, non-alcoholic steatohepatitis (NASH) has been on the rise, and has now become the most common cause of chronic liver disease in the United States (1).
Surgical management includes either hepatic resection (HR) or liver transplantation. Transarterial chemoembolization (TACE) and transarterial radioembolization (TARE) using Ytrium-90 (Y-90) have emerged as alternatives to surgery aimed at altering the natural history of the tumor (3). TACE is more commonly used than TARE due to its lower cost and wider availability. HCC has a strong propensity to invade liver vasculature, including portal vein, the hepatic veins or the retrohepatic inferior vena cava. Invasion of the portal vein is the most common form of macrovascular invasion in HCC with incidence around 10–60% at the time of diagnosis (4). HCC patients with portal vein tumor thrombosis (PVTT) have consistently been associated with extremely poor prognosis. This may be due to a combination of larger tumor size, worse liver function, and presence of numerous and more aggressive tumors (4). The mean survival for patients with untreated HCC who do not have PVTT is 24.4 months, which decreases to be as low as 2.4 months for those with PVTT (5-7).
Consensus guidelines from the American Association of Study of Liver Disease (AASLD), the Asian Pacific Association for the Study of Liver (APASL), and the European Association for the Study of Liver (EASL) recommend palliative treatment for HCC with PVTT (4). However, as surgical and interventional techniques continue to improve, a consensus has been unable to reached between the long and short-term oncological outcomes of HR and TACE. Through this meta-analysis, we aimed to systematically review and examine the literature comparing the survival benefits between HR and TACE in the treatment of HCC with PVTT.
Methods
Literature search and study selection
A comprehensive online search of MEDLINE, EMBASE, Google Scholar, SCOPUS and the Cochrane databases was performed for all published articles evaluating cancer specific overall survival (OS) following HR or TACE for patients with HCC with PVTT. The search was conducted using the following MESH terms: “Liver Resection” OR “Hepatectomy” OR “Liver Surgery” OR “Surgical Resection HCC” AND “Transarterial Chemoembolization” OR “TACE” AND “Resection Alone” AND “Hepatocellular Carcinoma” OR “HCC”. The related articles function was used to expand the search from each relevant study identified. All citations and abstracts identified were thoroughly reviewed. The latest search was performed on October 5, 2018. Bibliographies of retrieved papers were further screened for any additional eligible literature. This meta-analysis study was deemed exempt from ethics approval by the Institutional Review Board at University of Miami Miller School of Medicine ethical committee. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Outcomes of interest
Only studies reporting on survival comparing HR to TACE in patients with HCC with PVTT were included. Primary end-points of the study were 1-, 3- and 5-year OS rates between the two groups.
Inclusion criteria
To be included in the analysis, studies had to compare at least one of the survival rates above between patients who had HR or TACE for HCC patients with PVTT. When the same institution reported two studies, we either included the one of better quality, increased sample size, the most recent publication or both if the studies described different patient cohorts.
Exclusion criteria
Studies were excluded from analysis if they were either non-comparative studies or case series, also if the outcomes of interest were not reported for the two techniques, or there was significant overlap between authors, centers or patients’ cohorts evaluated in the published literature.
Data extraction
Two reviewers (C Ibrahim, N Parra) independently extracted the following data from each study: study characteristics (first author, year of publication, study interval, study design), population characteristics (number of patients included, demographics, tumor characteristics) and outcomes of interest (OS rates at 1-, 3-, 5-year).
Definitions
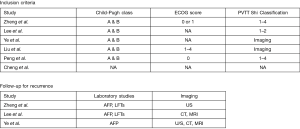
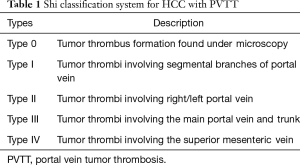
Several classification systems have been developed to classify and predict prognosis of patients with HCC with PVTT. The General Rules for the Clinical and Pathological Study of Primary Liver Cancer was developed and classified PVTT microscopically in 2006 (8). Later that year, Mei et al. divided PVTT into five grades based on location, proximal to distal (9). Finally, in 2007, the Shi Classification was developed which classifies PVTT into 4 different subtypes based on vessel invasion (Table 1) (10). The Shi Classification was the most widely used in the studies included in this analysis, and those that did not use this classification identified PVTT only by the presence of tumor thrombus in the portal vein seen on imaging.
Full table
TACE, a procedure in which the hepatic artery, the main blood supply in HCC, is blocked after chemotherapeutic agents (individually or combined) are given through the vessel. Sometimes, the anticancer drugs are attached to small beads that are injected into an artery that feeds the tumor. The beads block blood flow to the tumor as they release the drug. This allows a higher amount of drug to reach the tumor for a longer period of time, which may kill more cancer cells. TACE provides higher concentrations of therapeutic agents directly to the HCC and minimal systemic concentrations, thus minimizing systemic toxicity. Common chemotherapeutic agents given are doxorubicin (Adriamycin), cisplatin (Platinol AQ), and mitomycin (Mutamycin). All studies used similar TACE techniques with variations of the above chemotherapeutic agents.
Statistical analysis
All statistical analyses were performed using RevMan 5.3 (www.cochrane.org). Descriptive statistics were calculated for patients’ characteristics using mean, standard deviation, frequencies, and percentages. The method by Hozo et al. (11) was used for conversion to mean and standard deviation of variables reported as median and range. All P values of <0.05 (two-tailed) were considered statistically significant.
The primary end-point of the meta-analysis was 1-, 3- and 5-year OS between the two groups. Dichotomous variables were analyzed by computing the odds Ratio (OR) and corresponding 95% confidence interval (CI) as the summary statistic. Random-effects models were used weighted by the Mantel-Haenszel method (12,13). Statistical heterogeneity across studies was quantified using the χ2 (or Cochran Q statistic) and I2 statistic. The I2 statistic was derived from the Q statistic [(Q−df/Q) ×100], which provides a measure of the proportion of the overall variation attributable to heterogeneity between the studies. Homogeneity was considered absent if the Q statistic showed P<0.10, and the heterogeneity was considered significant when the I2 statistic exceeded 50%. Quality assessment of the observational studies was performed using the STROBE checklist (14).
Results
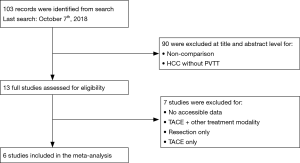
Our search yielded 103 publications. Based on title and abstract, only 13 studies fit our selection criteria and full-text was reviewed. Of the 13 selected for full-text review, 7 were excluded based on inclusion and exclusion criteria. Six retrospective cohort studies with a total of 1,320 patients were selected for inclusion in the meta-analysis (Figure 1); 526 patients (39.8%) underwent HR alone and 794 patients (60.2%) underwent TACE alone.
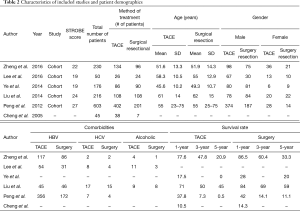
Mean age for the TACE group was 54±14.35 years compared to 55±15.73 years in the HR group. There was no significant difference between the two groups regarding age, sex, histopathology, and preoperative comorbidities, incidence of vascular invasion, intrahepatic metastases or incidence of bile duct invasion. Demographics of included studies are detailed in Table 2.
Full table
For diagnosis of HCC, the study by Zheng et al. used China’s Common Malignancy Specifications: Primary Liver Cancer (15), the studies by Lee et al., Peng et al., and Liu et al. used the European Association for the Study of the Liver (EASL) (16-18), and Liu et al. also used the American Association for the Study of Liver Disease (AASLD) (18). To define clinical staging, the study by Liu et al. used The Cancer of the Liver Italian Program (CLIP) and Barcelona Clinic Liver Cancer (BCLC) classification to define clinical staging (18). Zheng et al., Lee et al., and Peng et al. further classified the tumor using the Shi Classification for PVTT (15-17). Zheng et al. and Peng et al. treated patients with PVTT types 1–4, the study by Lee et al. only treated patients with PVTT type 1–2, and Ye et al. and Liu et al. classified PVTT based on imaging as detailed in Figure 2 (15-20).
The studies all used different ways of measuring and classifying severity of liver disease. The common measurements were Child Pugh Class, tumor number, tumor size, MELD score, and AFP level which have been added to the demographics (Table 2). Other measurements used by individual studies were cirrhosis, ascites and ECOG score. Overall, only one study reported a significant difference in CTP class (P≤0.001 with the surgical subgroup having a higher number of patients with CTP class A and B. This was also the only study that included CTP class C patients and these patients only received TACE). All other studies reported no significant difference in Child Pugh Class between surgery and TACE subgroups.
Three out of five studies reported a significant difference between the two subgroups in terms of the tumor number (Lee et al., Liu et al., Peng et al.). All three studies had a greater number of tumors in the TACE subgroup than in the surgery subgroup. Two out of five studies (Lee et al., Liu et al.) reported a significant difference between the two subgroups in regard to the tumor size with the surgical subgroup having overall smaller tumors. Only 3 studies reported MELD score, and one out of the three reported a significant difference between the two subgroups with the surgical subgroup having more patients with lower MELD scores. Finally, none of the studies reported a significant difference between surgical and TACE subgroups in terms of the AFP levels.
With regards chemotherapeutic agents used in TACE, Zheng et al. injected a combination of cisplatin, epirubicin, mitomycin and lipiodol into the vessel followed by embolization with a gelatin sponge (15). Peng et al. followed a similar protocol using lobaplatin, epirubicin, and mitomycin with lipiodol again followed by embolization with a gelatin sponge (17). Ye et al. used doxorubicin and cisplatin as the chemotherapeutic agents on gelfoam particles along with iodized oil (19). Lee et al. used doxorubicin alone as the chemotherapeutic agent and Liu et al. used adriamycin alone (16,18).
With regards to surgical resection, Ye et al. detailed that left hemihepatectomy was performed in 22 patients, right hemihepatectomy in 19 patients, left partial hepatectomy in 23 patients, right partial hepatectomy in 23 patients, partial median hepatectomy in 36 patients, and complete caudate lobe resection and extended left lateral segmentectomy in 3 patients. PVTT was removed in all patients who underwent hepatectomy. Hepatic vein tumor thrombus was simultaneously removed in 6 patients (3 with inferior vena cava thrombus and 3 with superior vena cava tumor thrombus), whereas extrahepatic bile conduct tumor thrombi were removed from 10 patients (19). Peng et al. detailed that only patients with normal or Child-Pugh class A liver function and ICG-R 15 <10% were offered major HR, which was defined as resection of 3 or more Couinaud’s segments of the liver. Selected patients with Child-Pugh class B liver function or ICG-R 15 >10% underwent surgery if the tumor was resectable by a minor HR, which was defined as resection of 2 or fewer segments of the liver. Patients with Child-Pugh class C liver function were not considered for resection (17). Zheng et al. did not detail only the portal thrombus management where PVTT that was located within the resected area was resected en bloc within the tumor. PVTT that protruded into the main portal vein beyond the resection line was extracted from the open stump of the portal vein. For cases with PVTT that extended into the main portal trunk with its primary branches on both sides of the vein, the main portal trunk was exposed and clamped distal to the PVTT (15). Liu et al. and Lee et al. did not describe type or extent of resection.
OS
All 6 studies included data of OS at 1 year. Only 5 out of 6 studies included OS at 3 years, and only 3 out of 6 studies included OS at 5 years. Median follow-up time range was from 1 to 5 years with an average of imaging every 3 months and monthly labs in the first postoperative year. The average median survival time for the resection group was 35 months compared to 17 months for the TACE group as stated in the 3 studies that reported this data. Meta-analysis of all included studies, with random effects model, showed longer OS in patients undergoing HR compared with TACE at 1-year (OR: 1.49, 95% CI: 1.16–1.92, P=0.002), 3 years (OR: 3.33, 95% CI: 1.55–7.12, P=0.002) and 5 years (OR: 3.91, 95% CI: 1.42–10.77, P=0.008) (Figure 3). There was no heterogeneity between studies at 1-year (I2=0%, P=0.53), but there was heterogeneity in the 3-year (I2=61%, P=0.05) and 5-year analysis (I2=81%, P=0.005).
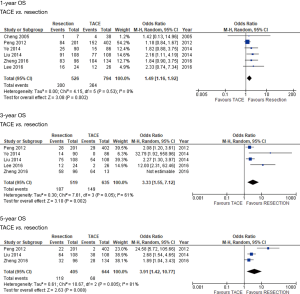
The study quality was assessed by STROBE checklist for observational studies. This showed that most of the studies were of relatively good quality with a mean score of 22. Subgroup analysis of studies with better quality (score >22) showed similar results to overall analysis confirming results and ruling out any skewing effect for lower quality studies.
Discussion
HCC with PVTT has classically been associated with a poor prognosis and only palliative treatment has been recommended by several consensus guidelines (4). HCC with PVTT has variable behavior depending on different factors. The etiology, extent of liver and vessel involvement, liver function, age of patient, and tumor size can all affect the OS. Until recently, the presence of tumor thrombus in the portal vein precluded HR (19). However, with advancements in surgical equipment and techniques as well as interventional procedures, HCC with PVTT is now a treatable condition with improved survival. Several studies in the last decade have reported on HR or TACE. In general, TACE has been increasingly offered as first-line treatment as these patients were being labeled as “unresectable”, however it is limited by availability, potential adverse effects, high costs, and limited efficacy (15,21). HR with parenchymal preservation can be safely performed in some selected cases. HR with thrombectomy may provide benefits such as decreasing portal vein pressure, and in turn, prevent intractable ascites and esophageal variceal bleeding. It also helps in the recovery of blood flow in the portal vein, improves liver function, and reduces the tumor burden, thereby improving quality of life and potentially survival (22,23).
Because the extent of tumor invasion of the portal vein can further affect prognosis, there have been multiple classification systems to categorize HCC with PVTT. The Shi Classification is the most widely used system to stratify the degree of PVTT. The increased survival with HR has been shown in cases with no involvement of the main trunk of the portal vein (or Shi Classification I and II) (15-17). PVTT involving the main trunk or superior mesenteric vein may lead to difficulties during the resection procedure. Any invasion to the venous wall may also lead to increased postoperative recurrence rates if any thrombi remain after surgery. Furthermore, tumor that has spread to the main portal trunk or SMV is an initial indication of poor biology and adverse outcomes (1). Vein obstruction can result in severe portal hypertension leading to overall decreased liver function, cirrhosis, and ultimately, to liver failure, esophageal varices, and intractable ascites (10). The fact that TACE addresses arterial blood supply of the tumor is compelling, however, in the inset of diminished portal flow, this may precipitate further liver tissue ischemia and poor liver function, thereby leading to worse survival (10,23). Ye et al. suggested that the increased benefits of surgical resection along the portal tributary is due to effectively eradicating the main solitary tumor reducing the risk of microportal invasion and intrahepatic metastasis (19,24). It is noteworthy that most patients with HCC with PVTT who underwent surgical resection are a highly selected group with adequate liver function and limited extent of tumor invasion prior to surgery. In an attempt to address this matter, two studies matched patients in order to eliminate that potential bias and better analyze the effects of treatment (17,18). Liu et al. used a propensity score analysis to compare TACE or surgical resection matching by baseline characteristics, disease burden, serum biochemistries, and performance status. In this study, HR had superior OS compared to TACE, further supporting our findings (18). Peng et al. also stratified patients by tumor number and the results continued to support HR as the superior treatment modality regarding survival for those with single tumors but not for those with multiple tumors. They concluded this to be because while complete ablation of tumor is beneficial in terms of decreased recurrence, liver function can be negatively affected by complicated or extensive surgery that may be required when the main portal vein trunk or the superior mesenteric vein are involved, decreasing OS (17).
We acknowledge that our meta-analysis has some important limitations. First, the data published by Cheng et al. (20) is written in the Chinese language. The numbers extracted for the 1-, 3-, and 5-year OS was obtained using Google translation. Furthermore, this meta-analysis included retrospective studies, which have inherent selection bias. For instance, patients undergoing HR possibly had superior liver function and smaller tumors deemed resectable. Regardless of the attempt to match TACE and HR groups, these biases are difficult to avoid. Moreover, the studies conducted by Zheng et al. and Peng et al. covered single centers in China with a high prevalence of individuals with HBV, stating that the results may not be reproducible in other locations around the world with different underlying liver diseases such as HCV or alcoholic cirrhosis. Also, pathological classification of those with PVTT who underwent TACE was not included, meaning that microvascular invasion was not assessed and may have contributed to tumor recurrence.
Conclusions
In conclusion, our analysis showed that surgical resection in patients with HCC and PVTT resulted in increased OS when compared to TACE. It is noteworthy that surgery is best suited for patients with no involvement of the main portal vein trunk, or Shi classification I and II only. Despite potential biases from included studies, we demonstrated that HR can be safely performed in selected patients with HCC with PVTT with satisfactory outcomes and should be included in consensus guidelines. Further investigation along with evaluation of short-term results using prospective studies are warranted.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This meta-analysis study was deemed exempt from ethics approval by the Institutional Review Board at University of Miami Miller School of Medicine ethical committee.
References
- Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog 2017;16:1. [Crossref] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat database: incidence — SEER 9 Regs research data. Nat Cancer Institute 2010. Available online: https://seer.cancer.gov/statfacts/html/all.html
- Schwartz M, Roayaie S, Uva P. Treatment of HCC in Patients Awaiting Liver Transplantation. Am J Transplant 2007;7:1875-81. [Crossref] [PubMed]
- Jiang JF, Lao YC, Yuan BH, et al. Treatment of hepatocellular carcinoma with portal vein tumor thrombus: advances and challenges. Oncotarget 2017;8:33911-21. [PubMed]
- Lu J, Zhang XP, Zhong BY, et al. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol 2019;4:721-30. [Crossref] [PubMed]
- Bae SH, Park HC. Editorial on combination treatment beyond sorafenib alone for hepatocellular carcinoma with portal vein tumor thrombosis. Transl Cancer Res 2017;6:S637-42. [Crossref]
- Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology 1999;29:62-7. [Crossref] [PubMed]
- Liver Cancer Study Group of Japan. The general rules for the clinical and pathological study of primary liver cancer. Jpn J Surg 1989;19:98-129. [Crossref] [PubMed]
- Mei MH, Chen Q, Yang JH. Clinicopathological staging of portal vein tumor thrombus in hepatocellular carcinoma and its significance. Chin J Hepatobiliary Surg 2006;12:374-7.
- Shi J, Lai EC, Li N, et al. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. Hepatobiliary Pancreat Sci 2011;18:74-80. [Crossref] [PubMed]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [Crossref] [PubMed]
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719-48. [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. Ann Intern Med 2007;147:W163-94. [PubMed]
- Zheng N, Wei X, Zhang D, et al. Hepatic resection or transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus. Medicine (Baltimore) 2016;95:e3959. [Crossref] [PubMed]
- Lee JM, Jang BK, Lee YJ, et al. Survival outcomes of hepatic resection compared with transarterial chemoembolization or sorafenib for hepatocellular carcinoma with portal vein tumor thrombosis. Clin Mol Hepatol 2016;22:160-7. [Crossref] [PubMed]
- Peng ZW, Guo RP, Zhang YJ, et al. Hepatic resection versus transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus. Cancer 2012;118:4725-36. [Crossref] [PubMed]
- Liu PH, Lee YH, Hsia CY, et al. Surgical resection versus transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. Ann Surg Oncol 2014;21:1825-33. [Crossref] [PubMed]
- Ye JZ, Zhang YQ, Ye HH, et al. Appropriate treatment strategies improve survival of hepatocellular carcinoma patients with portal vein tumor thrombus. World J Gastroenterol 2014;20:17141-7. [Crossref] [PubMed]
- Cheng SQ, Wu MC, Chen H, et al. Hepatocellular carcinoma with tumor thrombi in the portal vein. A comparison of therapeutic effects by different treatments. Zhonghua Zhong Liu Za Zhi 2005;27:183-5. [PubMed]
- Jianyong L, Jinjing Z, Wentao W, et al. Preoperative Transcatheter Arterial Chemoembolization for Resectable Hepatocellular Carcinoma: A Single Center Analysis. Ann Hepatol 2014;13:394-402. [Crossref] [PubMed]
- Luo J, Guo RP, Lai EC, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol 2011;18:413-20. [Crossref] [PubMed]
- Fan J, Zhou J, Wu ZQ, et al. Efficacy of different treatment strategies for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol 2005;11:1215-9. [Crossref] [PubMed]
- Arii S, Tanaka S, Mitsunori Y, et al. Surgical Strategies for Hepatocellular Carcinoma with Special Reference to Anatomical Hepatic Resection and Intraoperative Contrast-Enhanced Ultrasonography. Oncology 2010;78:125-30. [Crossref] [PubMed]