Numb chin syndrome secondary to leptomeningeal carcinomatosis from gastric adenocarcinoma
Introduction
Numb chin syndrome (NCS), or mental nerve neuropathy, was first described by Charles Bell in 1830 in a woman with breast cancer. Common features are pain, paresthesia, or hypoesthesia of the chin, lower lip, and gingiva along the distribution of the mental nerve, a division of the V3 branch of the trigeminal nerve (1-4).
NCS could be secondary to benign or malignant conditions. The most common causes are odontogenic, such as dental abscesses, anesthesia, local trauma, osteomyelitis, mandibular cysts and benign tumors. Systemic causes include sarcoidosis, amyloidosis, multiple sclerosis, HIV, diabetes mellitus, and vascular. Mandibular metastases are a common malignant cause of NCS (1,5). NCS could also be related to breast cancer, lymphoma, lung, thyroid, prostate, and colon cancer. The association of NCS with gastric carcinoma is rare (1,3,5,6).
Leptomeningeal carcinomatosis (LMC) is the dissemination and growth of malignant cells in the leptomeningeal space. LMC secondary to gastric cancer is infrequent (7).
We reviewed the case of a young Hispanic female who presented with left sided numbness of chin and headache associated with meningeal enhancement adjacent to the left temporal lobe. Initial work up was suggestive of sarcoidosis. Due to her clinical worsening and appearance of new gastro-intestinal symptoms, additional work up was performed and showed a poorly differentiated metastatic gastric adenocarcinoma.
Case report
A 27-year-old, right-handed, Hispanic female who presented to her primary care physician with complaints of left sided headache persisting for several days in February 2013. Headache was associated with numbness and tingling of the lower left gums and chin. She was diagnosed with Bell’s palsy and treated daily with oral prednisone and acyclovir.
Four days later, the patient returned with a new onset of blurred vision of the right eye and visual disturbances with persistent left-sided headache. An evaluation by ophthalmology revealed central serous chorioretinopathy and she was recommended continued treatment with oral prednisone.
Two weeks later, on February 28, the patient was admitted to hospital with complaint of worsening left fronto-temporal pulsatile headache and pain in the left ear. She denied nausea, photophobia, or phonophobia.
Physical examination showed that she was afebrile with stable vital signs. A left supraclavicular node was palpable. Otherwise, exam was unremarkable. Neurological examination revealed a right afferent pupillary defect with poor visual acuity of 20/200 in the right eye. Light touch and pinprick sensation was decreased in an area of the left chin. Mild left Bell’s palsy was noted. The rest of the exam was normal.
Laboratory workup revealed increased serum LDH with a level of 1,139 U/L (range, 313-618 U/L), erythrocyte sedimentation rate was elevated at a level of 33 (range, 0-20). ANA was positive, titer of 1/60, with a speckle pattern and anti-Sjögren syndrome-B antibodies were positive.
C-reactive protein, ACE levels, hepatitis panel, RPR, HIV, serum quantiferon gold test, borrelia burgdorferi antibodies, antineutrophil cytoplasmic autoantibody titers, anti-DsDNA antibodies, β2-microglobulin, complement levels were normal or negative.
Lumbar puncture showed increased opening pressure (27 cm H2O). CSF was clear and showed 12 RBC, 0 WBC, total protein was 31 mg/dL and glucose was normal. CSF IgG index was normal at 0.49 (normal up to 0.7) without oligoclonal bands. Myelin basic protein and ACE levels were negative. CSF cytology was negative for malignant cells by flow cytometry. Bacterial, fungal, and viral cultures were negative. Acid-fast bacilli were negative. VDRL was non-reactive. Viral panel, including CMV, EBV, VZV, HSV 1 and 2 DNA, were negative by PCR.
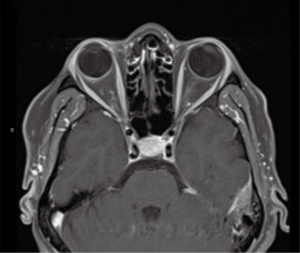
MRI of brain, with and without contrast, on admission to hospital showed focal abnormal meningeal enhancement adjacent to left temporal region (Figure 1) and related to the planum sphenoidale and right anterior clinoidal region. There was questionable enhancement of the intraorbital optic nerve sheaths and mild enlargement of the pituitary gland.
High resolution CT of chest showed mediastinal and abdominal lymphadenopathies. Pulmonary parenchyma had multifocal ground-glass appearance and nodular opacities, suggestive of sarcoidosis.
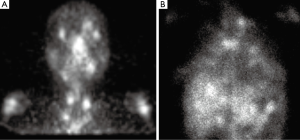
SPECT gallium scan showed increased uptake in the bilateral lacrimal and salivary glands known as the “Panda Sign” (Figure 2A), focal patchy areas of asymmetric uptake in the thoracic inlet, mediastinum and hilar regions and mid upper abdomen. Diffuse increased radiotracer activity was noted in both lungs (Figure 2B).
The patient was treated with IV Methylprednisolone 1 g daily for 5 days for presumptive sarcoidosis. Visual acuity and left chin numbness improved with steroids. She was discharged on oral prednisone 60 mg every other day and was scheduled for a lacrimal biopsy as outpatient.
Lacrimal biopsy was done the following week and reported as negative. Ten days after discharge on March 18, the patient was readmitted due to nausea, vomiting and jaundice. She developed obstructive cholestasis and pancreatitis. Endoscopic Retrograde cholangiopancreatography was performed and showed obstruction of the common bile duct and an abnormal friable and ulcerated area in the gastric mucosa extending to the gastro-esophageal junction. Gastric biopsy showed high grade invasive poorly differentiated adenocarcinoma (Figure 3A,B). By immunohistochemistry, HER-2 showed a strong membranous positivity (3+) (Figure 3C). Additionally, a pelvic mass biopsy was performed and confirmed metastatic gastric adenocarcinoma.

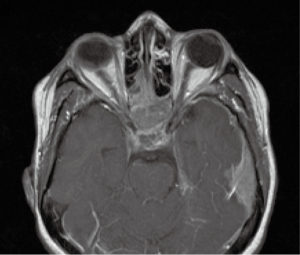
On April 2, she developed altered mental status, left CN VI palsy and decreased hearing on the left. Repeated MRI of brain, with and without contrast, showed significant interval worsening of nodular meningeal enhancement (Figure 4) and worsening of enhancing mass lesion in the sphenoid sinus extending through the skull base and into the sella turcica, cavernous sinuses and along the planum sphenoidale. There was another enhancing mass lesion within the left frontal sinus. She had new focal areas of restricted diffusion in the pons, left cerebellum, corpus callosum and bilateral cerebral hemispheres.
CA-125, CA 19-9, and CEA were elevated in blood. Lumbar puncture was recommended for confirmation of malignant cells in the CSF, but was refused by patient. At this time, the diagnosis of gastric adenocarcinoma with extensive metastatic disease to the liver, lungs, lymph nodes, bone, peritoneum, right eye and leptomeninges had been established. Treatment with FOLFOX was started. She developed sepsis and adrenal insufficiency and expired 85 days from onset of her neurological symptoms.
Discussion
NCS could be caused by the infiltration of the mental nerve from metastases or through compression of mental nerve by a lesion or tumor (1,4,5). Other causes could be metastasis to the mandible via lymphatic drainage, blood supply, or through neoplastic perineural infiltration (1). NCS is an uncommon presentation in patients with LMC resultant from leptomeningeal seeding by malignant cells (1,8).
LMC is more commonly seen in patients with leukemia, lymphoma, breast and lung cancers (6). Incidence of LMC from solid tumors is 3-8% with prevalence in gastric cancer of 0.16-0.69% (6,7,9). Irrespective of the site of origin, LMC is associated with a high mortality rate.
Gastric cancers are the third most common type of cancer worldwide. Approximately 650,000 deaths are reported per year, with the highest incidence in Asia and central Europe. The widespread increase in upper GI endoscopy has led to early detection and thus lowers stage gastric carcinomas. Furthermore, this trend has led to a better prognosis with a 5-year survival rate over 85%. However, development of LMC from gastric cancer yields a poor prognosis, often with neurological involvement (7). The majority of LMC cases from gastric cancer are histologically poorly differentiated adenocarcinoma with signet ring cell features (7,9).
The presence of the “Panda sign” in the SPECT gallium scan commonly suggests sarcoidosis. Other, less frequent causes (less than 4% of cases) are lymphoma, Sjögren’s syndrome, and AIDS (10,11).
In this case, extensive workup was suggestive of a systemic disease, like sarcoidosis. She had increased protein in the CSF with elevated opening pressure (12). Positive “Panda sign” in the gallium scan, multiple chest and abdominal lymphadenopathies, ground glass appearance of the lungs, and nodular meningeal enhancement were evidence supportive of this diagnosis. At the time of admission, the patient denied any other systemic symptoms including gastrointestinal complaints. Two weeks after discharge from hospital she started developing new symptoms that led to the diagnosis of metastatic HER2 positive gastric adenocarcinoma.
The diagnosis of gastric adenocarcinoma with increased CSF opening pressure and protein, multiple cranial nerve palsies, and brain MRI showing meningeal enhancement focally and along the base of the skull gave support to the diagnosis of LMC (13). LMC is typically diagnosed by lumbar puncture. In this case, first lumbar puncture was negative for malignant cells by flow cytometry and a second lumbar puncture was refused by patient. The sensitivity of CSF cytology for the diagnosis of LMC is limited with negative results in the first lumbar puncture in approximately 50% for all types of tumors (7,13-15). Although the brain MRI findings alone are not indicative of LMC, they are sufficient enough to make the diagnosis in the absence of a confirmatory CSF cytology in the presence of known cancer and appropriate clinical findings, as in our case (16).
A detailed cancer family history of the patient revealed that she had two maternal great aunts who died from gastric cancer in their 30s. She also had a family history of uterine cancer in relatives from both sides.
Physicians should be aware of the clinical features of NCS because it could be the first presenting symptom of malignancy, as in this patient. Appropriate work up could be done to facilitate an early diagnosis and management in order to improve patient outcome.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Laurencet FM, Anchisi S, Tullen E, et al. Mental neuropathy: report of five cases and review of the literature. Crit Rev Oncol Hematol 2000;34:71-9. [PubMed]
- Furukawa T. Charles Bell’s description of numb chin syndrome. Neurology 1988;38:331.
- Harris CP, Baringer JR. The numb chin in metastatic cancer. West J Med 1991;155:528-31. [PubMed]
- Tejani N, Cooper A, Rezo A, et al. Numb chin syndrome: A case series of a clinical syndrome associated with malignancy. J Med Imaging Radiat Oncol 2014;58:700-5. [PubMed]
- Baskaran RK. Krishnamoorthy, Smith M. Numb chin syndrome--a reflection of systemic malignancy. World J Surg Oncol 2006;4:52. [PubMed]
- Kim NH, Kim JH, Chin HM, et al. Leptomeningeal carcinomatosis from gastric cancer: single institute retrospective analysis of 9 cases. Ann Surg Treat Res 2014;86:16-21. [PubMed]
- Park KK, Yang SI, Seo KW, et al. A case of metastatic leptomeningeal carcinomatosis from early gastric carcinoma. World J Surg Oncol 2012;10:74. [PubMed]
- Peñarrocha Diago M, Bagán Sebastián JV, Alfaro Giner A, et al. Mental nerve neuropathy in systemic cancer. Report of three cases. Oral Surg Oral Med Oral Pathol 1990;69:48-51. [PubMed]
- Bulut G, Erden A, Karaca B, et al. Leptomeningeal carcinomatosis of gastric adenocarcinoma. Turk J Gastroenterol 2011;22:195-8. [PubMed]
- Prabhakar HB, Rabinowitz CB, Gibbons FK, et al. Imaging features of sarcoidosis on MDCT, FDG PET, and PET/CT. AJR Am J Roentgenol 2008;190:S1-6. [PubMed]
- Kurdziel KA. The panda sign. Radiology 2000;215:884-5. [PubMed]
- Stern BJ, Krumholz A, Johns C, et al. Sarcoidosis and its neurological manifestations. Arch Neurol 1985;42:909-17. [PubMed]
- Glass JP, Melamed M, Chernik NL, et al. Malignant cells in cerebrospinal fluid (CSF): the meaning of a positive CSF cytology. Neurology 1979;29:1369-75. [PubMed]
- Bernstein WB, Kemp JD, Kim GS, et al. Diagnosing leptomeningeal carcinomatosis with negative CSF cytology in advanced prostate cancer. J Clin Oncol 2008;26:3281-4. [PubMed]
- Glantz MJ, Cole BF, Glantz LK, et al. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer 1998;82:733-9. [PubMed]
- Clarke JL, Perez HR, Jacks LM, et al. Leptomeningeal metastases in the MRI era. Neurology 2010;74:1449-54. [PubMed]


