|
Case Report
Coexistence of gastrointestinal stromal tumour and colorectal
adenocarcinoma: Two case reports
Kinshuk Kumar1, Corwyn Rowsell2, Calvin Law3, Yoo-Joung Ko1
11Division of Medical Oncology, Sunnybrook Odette Cancer Centre, Toronto, Ontario, Canada; 2Division of Surgical Pathology, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada; 3Division of Surgical Oncology, Sunnybrook Odette Cancer Centre, Toronto, Ontario, Canada
Corresponding to: Yoo-Joung Ko, MD. Division of Medical Oncology, University of Toronto, Sunnybrook Odette Cancer Centre, 2075 Bayview Avenue, Toronto, ON M4N 3M5, Canada. Tel: 416-480-6100 ext 5847; Fax: 416-480-6002. Email: yoo-joung.ko@sunnybrook.ca.
|
|
Abstract
This paper reports two rare cases of patients with synchronous gastrointestinal stromal tumour (GIST) and colorectal adenocarcinoma (CRC) where adjuvant FOLFOX chemotherapy was administered concurrently with imatinib mesylate. The first case is a 67-year-old woman with a large gastrointestinal stromal tumour with metastasis masking a co-existing primary colon cancer, which was diagnosed after tumour response to imatinib mesylate. The second case presents a 61-year-old male with a primary colon cancer and a suspected metastatic lymph node, later confirmed to be a co-existing primary gastric GIST during colon surgery. While colorectal cancer is the third most common cause of cancer-related death in North America, the prevalence of GISTs remains rare. To date, very few cases of synchronous GIST and CRC adenocarcinoma have been reported in the literature. Although the coexistence of these two tumour types is rare, it is important to be aware of their disease patterns.
Key words
colonic neoplasms, gastrointestinal stromal tumors, neoplasms, multiple primary
J Gastrointest Oncol 2011; 2: 50-54. DOI: 10.3978/j.issn.2078-6891.2010.029
|
|
Case 1
In the fall of 2008, a previously well 67-year-old Caucasian
woman, presented with progressive fatigue over three
months accompanied by left lower abdominal pain. She
reported passage of “darker stools”; however, there was
no complaint of bright red blood per rectum or change in
stool shape. On physical examination, a minimally tender
palpable mass in the left lower quadrant was noted.
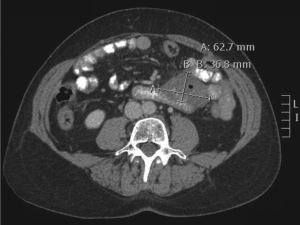
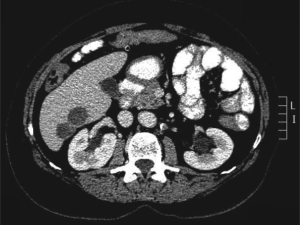
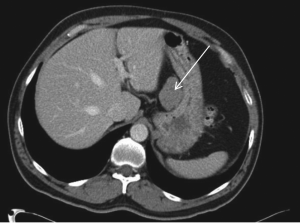
Computed tomography (CT) scan imaging revealed a
large abdominal mass (Fig 1) with multiple hypervascular
masses in the liver (Fig 2). The abdominal mass, with a
large area of internal necrosis, was intimately related to
the jejunum with minimal small bowel dilatation. One of the liver lesions in segment 4b was biopsied under
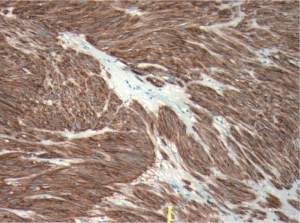
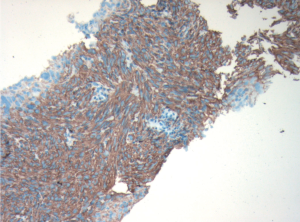
ultrasound guidance. Pathology revealed a spindle cell
tumour, which was strongly positive for CD117 and CD34
by immunohistochemistry (Fig 3). There were no mitotic
figures noted. The pathologic diagnosis was consistent
with metastatic gastrointestinal stromal tumour and in
December 2008, she was started on 400 mg of imatinib
mesylate per day.
Subsequently, follow-up CT imaging revealed significant
reduction of her primary GIST (Fig 4) as well as in the
hepatic metastases. The GIST decreased from its initial
size of 13.5 x 8.7 cm in November 2008 to 9.0 x 6.0 cm in
January 2009. The primary tumour continued to decrease
in size from 6.3 x 3.7 cm in June 2009 to 5.2 x 3.5 cm in
November 2009.
The CT scan in November 2009 revealed the presence
of a colonic mass with mesenteric lymphadenopathy.
The presence of the newly identified mass was confirmed
on colonoscopy, which revea led the presence of an
intraluminal mass at 80 cm from the anal verge. Biopsy of
this lesion revealed an invasive, moderately differentiated
adenocarcinoma of colonic origin.
After discussion at tumor board, a decision was made
to resect the primary colonic mass as well as the primary GIST. In December 2009, the patient underwent a left
hemicolectomy in addition to resection of the primary
GIST, which originated in the small bowel. The pathology
of the colonic mass revealed a moderately differentiated
adenocarcinoma with 7 out 12 lymph nodes involved.
The small bowel pathology revealed a spindle cell lesion
consistent with a GIST, which was positive for CD117 and
CD34. The Ki67 stain showed positivity in less than 1% of
tumour cells. The mitotic count was less than 1 per 50 High
Power Fields (HPF). The tumour showed large hypocellular
areas of hyalinization, an area of necrosis, and several areas
of hemorrhage as well as a focal hemangiopericytoma-like
pattern, consistent with treatment (imatinib mesylate)
effect. Of note, the laboratory findings did not include a preoperative
CEA, however, a CEA level was drawn shortly
after the surgery, measuring 2.5 ug/L.
She subsequently received 12 cycles of modif ied
FOLFOX-6 chemotherapy while remaining on imatinib for
her metastatic GIST. She did not experience any unexpected
toxicity from either the imatinib or chemotherapy and
remains well with continued regression of her liver
metastasis (GIST).
|
|
Case 2
A 61-year-old Caucasian gentleman presented with a change
in bowel habits and rectal bleeding in March 2009. He
reported no associated anorexia or weight loss. Colonoscopy
and biopsy revealed an adenocarcinoma at the splenic
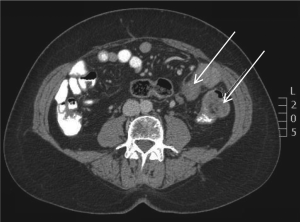
flexure. A staging CT scan also revealed a few subcentimeter
lymph nodes and a 5 cm mass at the gastrohepatic ligament
also suspected to be an enlarged metastatic lymph node (Fig
5).
In May 2009, at the time of surgery, the gastrohepatic
mass was resected. Once confirmed on a frozen section to
be a spindle cell tumour consistent with a GIST, a partial
gastrectomy was performed.
During the same operation, the patient also underwent a
left hemicolectomy. Final pathology revealed a 4 x 3.5 x 1.1
cm moderately differentiated adenocarcinoma with 4/22
lymph nodes being positive.
The gastric-based mass was a primary GIST measuring
5.5 cm. Histopathological examination revealed a spindle
cell lesion with a high mitotic index of 7 mitoses per 50 high
power fields (HPF) with negative resection margins. The
immunohistochemistry was positive for CD34 and CD117
(Fig 6) and negative for S100 and desmin. Ki67 stained 10%
of tumor cell nuclei. A pre-operative CEA level was normal
at 1.3 ug/L.
Post-operatively, he received 10 cycles of adjuvant
FOLFOX chemotherapy for his stage III colon cancer as
well as one year of adjuvant imatinib therapy for the GIST.
Imatinib (400 mg per day) was started after he had received
two cycles of modified FOLFOX-6.
|
|
Discussion
Defined as cellular spindle cell, epithelioid, or pleomorphic
mesenchymal tumour of the gastrointestinal (GI) tract,
the term gastrointestinal stromal tumour (GIST) was
introduced by Mazur and Clark in 1983 to differentiate
GISTs from leiomyomas ( 1, 2). The putative origin of these
tumours is believed to be the interstitial cells of Cajal, the
GI pacemaker cells ( 2-4). Approximately 95% of GISTs are
positive for expression of the KIT (CD117, stem cell factor
receptor) protein and as well as 70-80% of GISTs expressing CD34, the human progenitor cell antigen ( 2, 5). Although GISTs are the most common mesenchymal
tumours of the digestive tract, they remain rare. They
represent 0.1-3% of all GI cancers and have an incidence
of 10-20 cases/million ( 2, 4). Conversely, colorectal cancer
is the third most common cause of cancer-related death in
North America ( 6). While the incidence of synchronous
occurrence of other tumours with GISTs is on the rise,
there is no evidence of a common etiology ( 4, 7). Based on
the prevalence of both tumours, an incidental occurrence is
more likely. What remains important, however, is the need
to be aware of their coexistence. The first case outlines the presentation of a metastatic
small bowel GIST masking a colonic adenocarcinoma.
As the primary GIST decreased in size in response to
treatment with imatinib mesylate, the colonic mass and
enlarged mesenteric lymph node was unmasked. As lymph
node involvement with GIST is rare, the lymphadenopathy
was consistent with metastasis from a second primary
tumour. It also highlights that metastatic GIST should not
preclude the potential curative treatment of other secondary
cancers. The second case details a man with a primary
colonic neoplasm and an unidentified gastrohepatic mass
that was initially suspected to be a metastatic node but later confirmed to be a GIST. Given the atypical location of the
suspected lymph node, the patient underwent primary
surgery rather than systemic therapy. These cases highlight
the importance of being aware of second primary cancers
throughout the course of treatment for both colon cancer
and GISTs.
GISTs are most commonly found in the stomach
and small intestine. The coexistence of GISTs and
adenocarcinoma at two separate locations in the GI tract
is uncommon ( 7). Both colon cancer and GISTs are
infrequently associated with a genetic disposition and in
this report, neither patient reported a family history of any
malignancies. Surgery is the primary treatment modality for both
nonmetastatic GISTs and colon cancer ( 3). For metastatic
GIST, imatinib mesylate is the standard first-line treatment
( 8). Imatinib mesylate, a selective tyrosine kinase inhibitor,
has been shown to have a tumor response rate of greater
than 50% ( 3, 9). Continuous treatment with imatinib in the
metastatic setting is the standard treatment as interruptions
have been shown to result in rapid disease progression
( 10). Although surgery for patients with metastatic disease
is considered investigational, if the patient has disease
responsive to imatinib, surgical excision of a primary
tumor or an isolated metastasis that has progressed can be
associated with a good outcome ( 11). Treatment with imatinib in the adjuvant setting,
however, is now established as the standard of care for
those with resected primary GISTs ( 8). A phase III trial,
ACOSOG Z9001, was the first to demonstrate that one year
of imatinib as compared to placebo in the adjuvant setting,
is effective in decreasing recurrences. The trial included
713 patients with a resected GIST measuring at least 3 cm
in maximal diameter. Mitotic count was not an inclusion
criterion for this study. In this report, patient two had a
tumour greater than 3 cm and received adjuvant imatinib
therapy for one year consistent with the recommendations
of the major cancer societies ( 12, 13). Although adjuvant
imatinib is recommended for a minimum of one year, the
optimal duration of administration remains unknown.
The Intergroup EORTC 62024 trial is a randomized study
comparing two years of imatinib versus observation alone.
The Scandinavian Sarcoma Group (SSG) trial XVIII
is investigating three years versus one year of adjuvant
imatinib. Although both studies have completed accrual,
the results have not yet been presented. Hence, until the
results of these two studies are known, the recommended
duration of adjuvant treatment remains one year. A unique feature common to the two cases presented
is the concurrent treatment of adjuvant FOLFOX
chemotherapy with imatinib mesylate. Dexamethasone is a steroid that is commonly included as part of the antiemetic
regimen with a serotonin 5HT-3 antagonist in the
FOLFOX regimen. Both imatinib and dexamethasone are
metabolized by the cytochrome P450 (CYP450) isoenzyme
CYP3A4. Imatinib is a potent competitive inhibitor of the
CYP450 isoenzyme CYP3A4 while dexamethasone is an
inducer ( 14). There is a high possibility of a drug interaction
as the plasma concentration of imatinib may decrease when
administered with dexamethasone. While case two presents
a patient who received concurrent treatment for ten cycles
of FOLFOX, the patient in case one was administered
concurrent treatment for all twelve cycles. Although there
were no ill effects noted in either case, perhaps due to
the brief exposure of both dexamethasone and imatinib,
a more prolonged exposure of the two medications may
benefit from possible monitoring of plasma imatinib
levels especially in the setting of metastatic GIST (case
one). Modif ications to the treatment could include
increasing the dosage of imatinib, decreasing the dosage of
dexamethasone, or administering another anti-emetic in
lieu of dexamethasone.
|
|
Conclusion
There have been very few incidences of synchronous
colorectal cancer and GISTs reported in literature. Most of
the cases described were found due to other malignancies
or discovered incidentally during surgery ( 3, 5, 15). The two
cases presented above underline the importance of being
aware of this particular coexistence as well as the unlikely
metastatic spread of GIST to lymph nodes, development
of other primary tumours during treatment of metastatic
GIST, and the importance of a multidisciplinary approach
to cancer treatment.
|
|
References
- Mazur MT, Clark HB. Gastr ic stroma l tumors. Reappraisa l of
histogenesis. Am J Surg Pathol 1983;7:507-19.[LinkOut]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition,
clinical, histological, immunohistochemical, and molecular genetic
features and differential diagnosis. Virchows Arch 2001;438:1-12.[LinkOut]
- Melis M, Choi EA, Anders R, Christiansen P, Fichera A. Synchronous
colorectal adenocarcinoma and gastrointestinal stromal tumor (GIST).
Int J Colorectal Dis 2007;22:109-14.[LinkOut]
- Efstathios P, Athanasios P, Papaconstantinou I, Alexandros P, Frangisca
S, Sotirios G, et al. Coexistence of gastrointestinal stromal tumor
(GIST) and colorectal adenocarcinoma: A case report. World J Surg
Oncol 2007;5:96.[LinkOut]
- Kosmidis C, Efthimiadis C, Levva S, Anthimidis G, Baka S, Grigoriou
M, et al. Synchronous colorectal adenocarcinoma and gastrointestinal stromal tumor in meckel's diverticulum; an unusual association. World
J Surg Oncol 2009;7:33.[LinkOut]
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J
Clin 2010;60:277-300.[LinkOut]
- Gopal SV, Langcake ME, Johnston E, Salisbury EL. Synchronous
association of small bowel stromal tumour with colonic
adenocarcinoma. ANZ J Surg 2008;78:827-8.[LinkOut]
- DeMatteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW,
Demetri GD, et al. Adjuvant imatinib mesylate after resection of
localised, primary gastrointestinal stromal tumour: a randomised,
double-blind, placebo-controlled trial. Lancet 2009;373:1097-104.[LinkOut]
- Spinelli GP, Miele E, Tomao F, Rossi L, Pasciuti G, Zullo A, et
al. The synchronous occurrence of squamous cell carcinoma and
gastrointestinal stromal tumor (GIST) at esophageal site. World J Surg
Oncol 2008;6:116.[LinkOut]
- Le Cesne A, Ray-Coquard I, Bin Bui N, Adenis A, Rios M, Duffaud
F, et al. Time to onset of progression after imatinib interruption
and outcome of patients with advanced GIST: Results of the BFR14 prospective french sarcoma group randomized phase III trial [abstract].
J Clin Oncol 2010; s28:10033.
- Mussi C, Ronellenfitsch U, Jakob J, Tamborini E, Reichardt P, Casali
PG, et al. Post-imatinib surgery in advanced/metastatic GIST: Is it
worthwhile in all patients? Ann Oncol 2010;21:403-8.[LinkOut]
- Nccn.org [Internet]. Washington: National Comprehensive Cancer
Network. c2010 [cited 2010]. Available from: http://www.nccn.org/.[LinkOut]
- Casa li PG, Jost L, Reichardt P, Schlemmer M, Blay JY, ESMO
Guidelines Working Group. Gastrointestinal stromal tumours: ESMO
clinical recommendations for diagnosis, treatment and follow-up. Ann
Oncol 2009;20:s64-7.[LinkOut]
- Scripture CD, Figg WD. Drug interactions in cancer therapy. Nat Rev
Cancer 2006;6:546-58.[LinkOut]
- Tzilves D, Moschos J, Paikos D, Tagarakis G, Pilpilidis I, Soufleris K,
et al. Synchronous occurrence of a primary colon adenocarcinoma and
a gastric stromal tumor. A case report. Minerva Gastroenterol Dietol
2008;54:101-3.[LinkOut]
Cite this article as:
Kumar K, Rowsell C, Law C, Ko Y. Coexistence of gastrointestinal stromal tumour and colorectal
adenocarcinoma: Two case reports. J Gastrointest Oncol. 2011;2(1):50-54. DOI:10.3978/j.issn.2078-6891.2010.029
|