
Proton beam re-irradiation for gastrointestinal malignancies: a systematic review
Introduction
Radiotherapy (RT) plays an important role in the management of many gastrointestinal (GI) cancers, and is used in the palliative, neoadjuvant, adjuvant, and definitive setting for patients with esophageal (1), gastric (2), pancreatic (3,4), hepatobiliary (5,6), rectal (7), and anal cancers (AC) (8).
Even with advanced RT, systemic therapy, and surgical options available, many patients will experience locoregionally recurrent (LRR) disease, or develop a new primary cancer within a previously-irradiated field. Retreatment by any modality presents a challenge for both patients and physicians in regards to how best to deliver treatment effectively and safely. In this setting, management options frequently include one or a combination of RT, systemic therapy, surgery, ablative therapy, radionuclide therapy, clinical trials, or supportive care. From an RT standpoint, photon re-irradiation options exist. While there are few published randomized data in the re-irradiation setting, in recurrent head and neck (HN) cancer, a randomized trial assessing the role of post-operative re-irradiation with chemotherapy following salvage surgery found that patients who received post-operative CRT [with conventional RT or three-dimensional conformal RT (3D-CRT)] after salvage surgery experienced significantly-improved disease-free survival (DFS), as compared to patients who underwent salvage surgery alone, though at the expense of increased acute and late toxicity (9).
In GI malignancies, non-randomized data exist for photon re-irradiation with older techniques, with mixed results. A series of 10 patients that received photon re-irradiation [non-intensity modulated RT (IMRT)] for recurrent EC found that RT produced a tumor response in 50% of patients 3 months from the end of re-irradiation, with a median follow-up of 4.9 months, albeit with development of grade 5 tracheoesophageal fistulae in 3 patients (10). A prospective study of 72 patients with locally recurrent (LR) unresectable RC who received re-irradiation with hyperfractionated 3D-CRT and concurrent capecitabine found an overall response rate and clinical benefit rate of 59.7% and 93.1%, respectively, with grade 3–4 diarrhea and granulocytopenia in 9.7% and 8.3%, respectively, and a 1.4% rate of small bowel obstruction (SBO) (11).
Given the potential for re-irradiation to control recurrent or new primary disease, there has been interest in applying more modern RT techniques to minimize the associated toxicity. Within photon re-irradiation, IMRT has been used to re-irradiate LR RC, with successful palliation in 55.6% of patients, 61.3% and 47.3% 1- and 2-year LC, and 32.3% and 3.2% grade 2 and 3 acute toxicity rates, respectively (12). Within IMRT, groups at Harvard (13), Stanford, and Johns Hopkins have studied the use of stereotactic body RT (SBRT) for re-irradiation for recurrent PC, with LC ranging from 62–78% and a 6–7% risk of SBO (14).
With evidence that modern photon re-irradiation techniques may permit efficacious and safe delivery of RT for recurrent or new primary disease, there has also been interest in the use of particle therapies, like carbon-ion therapy (CIT) and proton-beam therapy (PBT) to further lessen the toxicity of RT and improve the therapeutic ratio. A prospective report of CIT to re-irradiate a variety of cancers (over one-third of which were RC or HCC) demonstrated 71% and 60% 1- and 2-year LC, with no grade ≥2 acute toxicities, 18% grade 3 late toxicities, and no grade ≥4 late toxicities (15). Another report of CIT re-irradiation, in 19 patients with LR RC, found 79% 1-year LC, with no grade ≥3 toxicities at a median follow-up of 7.8 months (16).
PBT is another particle therapy that has garnered recent interest, given the favorable properties of PBT dose deposition. PBT can deliver equivalent doses to targets as would photons while sparing integral dose to OARs, which could potentially reduce toxicity (17). PBT has been used clinically in the up-front setting to treat primary brain tumors (18), HN cancer (19,20), thoracic malignancies (21-23), breast cancer (24,25), gynecologic cancers (26), prostate cancer (27,28), and GI malignancies (29) with reasonable efficacy and safety. PBT has been used in the re-irradiation setting to treat adult and pediatric central nervous system tumors (30,31), HN cancer (32,33), thoracic malignancies (34,35), breast cancer (36), gynecologic cancers (37), and GI malignancies (38), also with reasonable efficacy and safety. At present, a dedicated review of PBT re-irradiation for GI malignancies has not been published. Such a review could potentially better familiarize clinicians with the use of PBT re-irradiation in this setting, better inform their decision-making discussions with patients and colleagues, and pave the way for further study. Herein, we provide a systematic review of the published experiences of PBT re-irradiation in patients with recurrent or second primary GI malignancies.
Methods
We conducted our systematic review as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement protocol (39). Inclusion criteria consisted of any English language published literature reporting on patients with recurrent or second primary GI malignancies treated with PBT re-irradiation (e.g., PBT within the field of a prior RT course). Comparison and/or outcome measures assessed were any cancer-control outcomes, survival outcomes, symptom-relief outcomes, and/or acute or late toxicities. PubMed was the primary information source (most recently searched on July 21, 2019, without other date constraints), while a minority of articles were identified from within the text of articles found in PubMed. Search terms in PubMed included combinations of the words “proton”, “beam”, “therapy”, “radiation”, “radiotherapy”, “re-irradiation”, “re-treatment”, and “repeat”, with combinations of hyphenation to maximize search output.
Using the search criteria above and in-text article identification, 373 articles were identified (Figure 1). A total of 10 duplicate articles were excluded, as they represented initial reports of subsequently updated published cohorts. Of the remaining 363 articles, 352 were excluded, as they did not fully meet the inclusion criteria above, as assessed by 3 authors. Commonly, the reasons for exclusion were non-PBT re-irradiation, receipt of only one RT course, non-GI malignancies receiving re-irradiation, or pre-clinical work (physics, dosimetry, or cell biology studies without disease/symptom-control or toxicity outcomes), among others. Of the remaining 11 articles, 4 articles were ineligible due to being review articles, thus establishing our final inclusion of 7 primary reports for quantitative synthesis. Within these 7 reports, we manually extracted data from the published articles regarding initial cancer-directed treatments, with particular attention to RT, as well as subsequent cancer-directed treatments, with particular attention to PBT re-irradiation and any concurrent therapies administered. We then extracted the comparison and outcome measures described above, as well as any accompanying dosimetric data, when present. Given the substantial variability in disease site, study size, and methodologies used in the 7 studies included, neither meta-analysis nor other comparative statistical methodologies were performed.
Results
Esophageal cancer
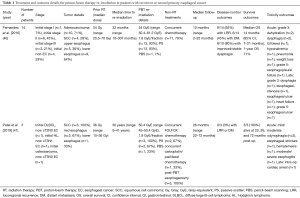
Two published experiences of PBT re-irradiation for EC are displayed in Table 1. The first is a 14-patient prospective feasibility trial that enrolled patients with recurrent or second primary EC with Karnofsky performance statuses (KPS) >60, life expectancies >3 months, and ≥3-month intervals from prior RT courses (40). During the initial courses of RT, patients were treated for stage I (7%), stage II (43%), or stage III (21%) EC, or non-EC (29%) that received RT in the subsequent field of their EC PBT. Four (29%) previously underwent esophagectomy. The median prior RT dose was 54 Gy (range, 25.5–70 Gy). Patients who met the above criteria then received PBT re-irradiation to gross disease to a median re-irradiation dose of 54 GyE (range, 50.4–61.2 GyE) in 1.8 GyE fractions for all but one, and to a cumulative dose of 109.8 GyE (range, 76–129.4 GyE), at a median interval of 32 months (range, 10–307 months) from their prior RT courses. PBT technique was 2–3-beam passive scattering (PS) in 13 (93%) patients and pencil-beam scanning (PBS) in 1 (7%) patient, with either cord-sparing anterior fields (with anterior bolus as needed) or heart/lung-sparing posterior fields based upon tumor location. Two (14%) patients received 30% and 14% of their treatments with IMRT as a result of pleural effusion formation and PBT downtime, respectively. Most patients (79%) received concurrent chemotherapy with PBT, most commonly 5-fluorouracil- (5-FU) based (73%). The majority had adenocarcinoma (71%) and lower esophageal tumors (64%). The primary endpoints included feasibility and acute toxicity. The secondary endpoints were late complications, disease control, and OS. At a median follow-up of 10 months (range, 2–25 months) from the start of re-irradiation, 8 of 10 patients that presented with dysphagia had improved or stable symptoms. LRR and DM were observed in 9 (64%) and 6 (43%) patients, respectively. The median and 1-year OS were 14 months (95% CI, 7–21 months) and 71%, respectively. Acute grade ≥3 toxicities included grade 3 dehydration (n=2, 14%), dysphagia requiring feeding tube or stent (n=2, 14%), GI bleed (n=1, 7%), hyponatremia (n=1, 7%), pneumonia (n=1, 7%), weight loss (n=1, 7%), and grade 5 esophagopleural fistula (n=1, 7%, favored to be due to tumor progression rather than PBT, as this occurred following the second fraction). Late grade ≥3 toxicities included grade 3 dysphagia (n=1, 7%), esophageal stenosis (n=1, 7%), esophageal ulcer (n=1, 7%), heart failure (n=1, 7%), and grade 5 esophageal ulcer (n=1, 7%, favored to be due to LR/persistence rather than PBT).
Full table
Patel et al. (41) present a retrospective case series of 3 patients who received thoracic RT as part of treatment for prior malignancies and subsequently developed esophageal squamous cell carcinoma (SCC), for which they underwent PBT re-irradiation. The first patient previously received involved-field RT to a dose of 36 Gy with 6 cycles of R-CHOP for mediastinal diffuse large B-cell lymphoma, in addition to additional systemic therapy and stem-cell transplant, complicated by pulmonary fibrosis and cardiomyopathy. Five years after his initial RT course, he was found to have cT2N0 esophageal SCC of the mid-esophagus. The second patient previously received mantle/para-aortic RT to a dose of 36 Gy for Hodgkin lymphoma, complicated by coronary artery disease and aortic stenosis. Thirty years after her initial RT course, she was found to have a cT3N1 esophageal SCC of the mid-esophagus. The third patient previously received whole-lung RT to 15 Gy for metastatic osteosarcoma, with multiple cycles of doxorubicin-based chemotherapy, complicated by cardiomyopathy and arrhythmias requiring a pacemaker. Forty-one years after her initial RT course, she was found to have a cT3N0 esophageal SCC of the lower esophagus. All 3 patients received PBT re-irradiation to an initial dose of 45 GyE with a clinical tumor volume (CTV) expansion of 3.5 cm superior/inferior and 1.5 cm circumferentially in 1.8 GyE fractions, with 2 (67%) receiving a boost of 5.4 GyE to primary disease. The median cumulative dose was 81 GyE (range, 65.4–86.4 GyE). PBT technique was 1–2-beam posterior-oblique PS and 1-beam posterior-anterior PBS in 2 (67%) and 1 (33%) patients, respectively. All patients received concurrent chemotherapy (67% FOLFOX, 33% carboplatin/paclitaxel), and underwent esophagectomy following RT (67% initially unplanned). At a median follow-up of 26 months (range, 22–72 months) from esophagectomy, all 3 patients were alive, without evidence of recurrent or metastatic disease. Acute toxicities included mild/moderate odynophagia (n=2, 67%), esophageal stricture requiring balloon dilation (n=1, 33%), hematemesis (n=1, 33%), and moderate/severe esophagitis (n=1, 33%). The only late toxicity reported was intra-operative cardiac arrest and cardiogenic shock during esophagectomy, requiring extracorporeal membrane oxygenation, from which he recovered. In addition, comparison volumetric-modulated arc therapy (VMAT) plans were designed, with similar target and OAR constraints, which showed reduced OAR doses and equal target coverage with PBT, with the exception of the lung V20Gy/V30Gy parameters in the 2 patients treated with PS.
Pancreatic cancer
One published experience of PBT re-irradiation for PC is displayed in Table 2 (42). This was a 15-patient report that included patients treated on a prospective safety and feasibility trial (n=13) that enrolled patients with isolated LR PC with KPS >60, life expectancies >3 months, and ≥3-month intervals from prior RT courses, or on an institutional PBT registry who met the aforementioned criteria (n=2). During the initial courses of RT, patients were treated for resectable (67%), borderline resectable (20%), or unresectable (13%) PC of the pancreatic head (67%), body (20%), or tail (13%). Most patients initially underwent resection with either neoadjuvant (27%) or adjuvant CRT (60%), while the remainder underwent definitive CRT (13%). The median prior RT dose was 50.4 Gy (range, 30–59.4 Gy). Patients who met the above criteria then received PBT re-irradiation to gross disease and high-risk regions with an internal target volume (ITV) for respiratory motion and 0.5–1 cm planning target volume (PTV) margin to a median re-irradiation dose of 59.4 GyE (range, 37.5–59.4 GyE) in 1.8 GyE fractions for all but one, to a median CTV/ITV volume of 71 cc (range, 15–200 cc), at a median interval of 26.7 months (range, 7–90 months) from their prior RT courses. PBT technique was 2–3-beam PS based upon tumor location and prior OAR doses. One (7%) patient received 35% of RT with VMAT to optimize duodenal sparing. Most patients (67%) received concurrent chemotherapy with PBT, all 5-FU-based. PBT targets included nodal regions in 73% (celiac, n=6, 55%; aortocaval, n=2, 18%; porta hepatis, n=2, 18%; superior mesenteric, n=1, 9%) and/or surgical bed/progressive primary disease after definitive CRT in 47% of patients. At a median follow-up of 15.7 months (range, 2–48 months) from the start of re-irradiation, 6 of 7 patients that presented with symptomatic, painful LR experienced pain palliation. LRR (in-field) and DM were observed in 4 (27%) and 13 (87%) of patients, respectively, with one of the LR’s occurring in a patient treated to a palliative RT dose. The median and 1-year OS were 16.7 months (95% CI, 4.7–36 months) and 67%±12%, respectively. Patients that received concurrent chemotherapy had significantly longer median OS (22.8 months) than those that did not (7.6 months, P=0.003). Of the 7 patients with elevated cancer antigen 19-9 levels prior to PBT, 5 (71%) experienced decreased levels after PBT. Acute grade he 7 patients with elevgrade 3 anorexia (n=1, 7%), fatigue (n=1, 7%), grade 4 bleeding duodenal ulcer (n=1, 7%), and grade 5 small bowel perforation following gastric outlet obstruction (n=1, 7%, favored to be due to disease progression and stent placement rather than PBT). There were no late grade ≥2 toxicities.

Full table
Liver tumors
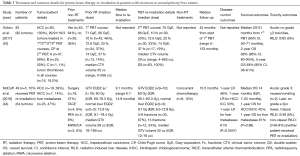
Two published experiences of PBT re-irradiation for liver tumors are displayed in Table 3. The first is an 83-patient (92 tumors) retrospective report of PBT re-irradiation in patients with recurrent or second primary HCC (43). In this report, patients received PBT re-irradiation if it was deemed that non-RT treatments would not be feasible, or if the patient refused non-RT treatments. The median initial RT dose was 71 GyE (all delivered with PBT), with regimens most commonly consisting of 66 GyE in 10 fractions (n=42, 46%), 72.6 GyE in 22 fractions (n=34, 37%), or 74 GyE in 37 fractions (n=13, 14%), to a median CTV volume of 35 cc (range, 3–936 cc). 64% of patients received initial non-RT treatments as well. A total of 91, 16, and 3 tumors were treated in the 2nd, 3rd, and 4th PBT courses. Patients received PBT re-irradiation to a median 2nd course dose of 70 GyE (all double-scatter technique), with regimens most commonly consisting of 66 GyE in 10 fractions (n=30, 33%), 72.6 GyE in 22 fractions (n=30, 33%), or 74 GyE in 37 fractions (n=17, 19%), to a median CTV volume of 29 cc (range, 4–665 cc). At a median follow-up of 45 months (range, 5–153 months) from the start of the initial PBT course, the median OS was 61 months (95% CI, 50–71 months), and 2- and 5-year OS were 88% (95% CI, 80–95%) and 49% (95% CI, 38–61%), respectively. There were no acute grade ≥3 toxicities and no cases of radiation-induced liver disease (RILD).
Full table
McDuff et al. (44) present a retrospective report of 49 patients (64 tumors) who received re-irradiation to the liver for recurrent or second primary liver tumors. Of note, only 5 patients (10%) received re-irradiation using PBT. In this cohort, patients with HCC received re-irradiation if they were felt to be suboptimal candidates for other local therapies. Patients with IHCC received re-irradiation if tumor anatomy precluded resection or they progressed after initial adjuvant RT. Patients with liver metastases received re-irradiation if they had oligometastatic disease or limited local progression with controlled systemic disease. During the initial courses of RT, patients were treated for HCC (39%), IHCC (14%), or liver metastases (47%). Initial non-RT local treatments consisted of surgery (37%), transcatheter arterial chemoembolization (8%), radiofrequency ablation (8%), and microwave ablation/cryotherapy (6%). The median prior RT doses to the gross tumor volume (GTV) [equivalent dose in 2 Gy fractions (EQD2), α/β=10] and normal liver (EQD2, α/β=3), were 60 Gy [interquartile range (IQR), 60–76.3 Gy] and 13.9 Gy (IQR, 9.1–19.4 Gy), respectively. The median prior GTV volume was 59 cc (IQR, 18–189 cc). Patients who met the above criteria then received re-irradiation to the GTV (median volume, 25 cc, IQR, 12–76 cc), with an ITV for target motion, a 0–0.5 cm margin for CTV, and an additional 0.5 cm margin for PTV, at a median of 9.1 months (range, 6.7–14.9 months) from their prior RT courses. The median re-irradiation doses to the GTV (EQD2, α/β=10) and normal liver (EQD2, α/β=3) were 60 Gy (IQR, 59.1–83.3 Gy) and 9.7 Gy (IQR, 6.5–12.9 Gy), respectively, delivered most commonly in 5–6 fractions (67%) or 15 fractions (24%), occasionally with concurrent chemotherapy (12%). The median follow-up was 10.5 months. The 1-year LR rate was 46% for the whole cohort, 33% for patients with HCC/IHCC, and 61% for patients with liver metastases (P=0.0047). The median OS for the whole cohort was 14 months (IQR, 7–22 months), with 1-year OS for patients with HCC/IHCC and liver metastases of 45% and 61% (P=0.86), respectively. Acute grade ≥3 toxicities included grade 3 nausea/vomiting (n=2, 4%). There were no late grade ≥3 toxicities or cases of classic RILD. There were 2 cases (4%) of non-classic RILD, but neither patient received PBT re-irradiation.
Rectal cancer
One published experience of PBT re-irradiation for RC is displayed in Table 4 (45). This was a 7-patient report that included patients treated on a prospective toxicity and feasibility study with LR RC with KPS KPS ≥60, life expectancies ≥3 months, and ≥3 months since prior RT courses. This was a 7-patient report that included patients treated on a prospeDuring the initial courses of RT, patients were treated for stage I (14%), II (29%), III (43%), or IV (14%) RC, with 5 (71%) patients having colostomies prior to PBT. The median prior RT dose was 50.4 Gy (range, 44–86.4 Gy), with 2 (29%) patients receiving 2 RT courses prior to PBT. Patients who met the above criteria then received PBT re-irradiation to gross recurrent disease or tumor bed with a margin for microscopic disease, expanded by 0.5–1 cm for PTV from CTV to a median re-irradiation dose of 61.2 GyE (range, 45–64.8 GyE) in 1.8 GyE fractions, to a median CTV volume of 246 cc (range, 82.9–514.2 cc), at a median interval of 39 months (range, 9–93 months) from their prior RT courses. The median cumulative RT dose was 109.8 GyE (range, 95.4–151.2 GyE). PBT technique was typically 2–3-beam (86%) or 1-beam (14%) PS. Most patients (86%) received concurrent chemotherapy with PBT, all 5-FU-based. At a median follow-up of 19.4 months (range, 4.9–30.7 months), 6 of 6 patients that presented with painful LR experienced either complete or partial pain palliation, LRR/local progression and DM were observed in 3 (43%) and 1 (14%) patient, respectively, and 4 (57%) were alive. Acute grade ≥3 toxicities included grade 3 diarrhea (n=3, 43%) and abdominal pain (n=1, 14%), which all resolved. Late grade diarrhea (n=3, 43%) and abdominal pain (n=1, 14%), which all reentero-vaginal fistula (n=1, 14%). Of note, one of the bowel obstructions was post-operative, following attempted resection of residual tumor following PBT, and the same patient developed the observed entero-vaginal fistula, favored to be due to tumor progression into the vagina. In addition, comparison IMRT plans were designed, with similar target and OAR constraints, which showed statistically significant decreases in bowel V10 and V20, and in the dose to 200 and 150 cc of bowel, with PBT as compared to IMRT.

Full table
AC
One published experience of PBT re-irradiation for AC is displayed in Table 5 (46). In this case report, the patient previously received low dose rate brachytherapy (125I, minimum peripheral dose 145 Gy) to the prostate for low-risk prostate cancer. Nine years after brachytherapy, he was found to have pT2N0 SCC of the anal canal, resected via transanal excision with close margins. He was initially felt not to be a candidate for RT given his prior RT, and was surveilled. The following year, he was found to have recurrent, bulky, cT3N0 AC, spanning from 3–8 cm from the anal verge. He was offered abdominoperineal resection, but refused, and was then offered PBT re-irradiation, concurrently with chemotherapy. PBT was delivered via sequential boost to a dose of 45 GyE in 25 fractions to the peri-prostatic tissue, 50.4 GyE in 28 fractions to the inguinal, internal iliac, and perirectal lymph nodes, and 59.4 GyE in 33 fractions to gross tumor. The CTV to PTV expansion was 0.5 cm for the 45 and 50.4 GyE dose levels, and 0.3 cm for the 59.4 GyE dose level. PBT technique was PBS utilizing opposed lateral beams, matched to 2 16 MeV electron beams prescribed to the 90% isodose line. This arrangement was used, rather than standard PBT posterior obliques, to limit dose and toxicity to the region of prior RT in the prostate and prostatic urethra. Dosimetrically, all genitalia and femoral head constraints were achieved. The cumulative small bowel, prostate, and prostatic urethra maximum doses were 58 GyE (54 GyE desired), 52.7 GyE (45 GyE), and 25.5 GyE (25 GyE), respectively. Follow-up, disease-control, and toxicity outcomes were not reported.

Full table
Discussion
Herein, we provide the first systematic review of PBT re-irradiation in GI malignancies. Based upon the studies described, PBT re-irradiation for patients with recurrent or second primary GI cancers appears to be a promising option in terms of efficacy and safety, though with the need for additional data, with longer follow-up and larger samples to further validate these early experiences.
The ability of PBT re-irradiation to provide symptomatic palliation was demonstrated across many of the GI sites reported. In EC, Fernandes et al. (40) found that of the 10 patients who presented with dysphagia from recurrent tumor, 4 had complete and 3 had partial resolution of symptoms, while 1 had stable symptoms. In PC, Boimel et al. (42) found that 6 of the 7 patients who presented with painful recurrent disease reported resolution of pain following PBT re-irradiation. In RC, Berman et al. (45) found that all 6 patients who presented with painful recurrent disease also reported resolution of their pain following PBT re-irradiation. While in some scenarios, it is possible that re-irradiation will be part of a curative-intent approach, it is also the unfortunate reality that even with PBT, cure may not be possible, and that the treatment intent may be durable LC and symptom palliation. It is encouraging that across multiple disease sites, PBT re-irradiation was effective in providing palliation.
In terms of disease-control, survival, and toxicity outcomes, while understanding that it is difficult to compare experiences between different modalities and studies, PBT re-irradiation for GI malignancies appears promising. In recurrent EC, Fernandes et al. (40) reported that 36% and 57% of patients remained free of LRR and DM at a median follow-up of 10 months following re-irradiation, with a median OS of 14 months, in an adenocarcinoma-predominant (71%) cohort, at a median of 32 months from their initial RT courses. In second primary EC, Patel et al. (41) found that all 3 patients remained free of LRR and DM, and alive, at a median follow-up of 26 months after re-irradiation, in an entirely SCC cohort, at a median of 30 years from their initial RT courses. Within these 2 studies, there is clear heterogeneity, as nearly all patients in the Fernandes study were treated for EC initially, to higher prior RT doses than those in Patel’s study, and with primarily adenocarcinoma histologies. In Patel’s report, the 3 initial RT courses were for non-GI malignancies, had maximum doses of 36 Gy, had as many as 41 years between courses, and were all SCC. This heterogeneity speaks to the variety of scenarios in which PBT re-irradiation can play a role in achieving cure or palliation in recurrent and second primary EC. Treatment was relatively well-tolerated, being administered with concurrent chemotherapy in the majority of patients in both of the above studies, with reasonable grade 3 toxicities, and 2 grade 5 toxicities, both favored to be due to progressive disease, rather than PBT. In addition, in Patel’s report, treatment was tolerated well enough that each patient, 2 of whom were felt to be medically inoperable prior to re-irradiation, all had curative-intent surgeries. One prior re-irradiation experience for EC, with 3D-CRT, reported tumor response rates of 50% at 3 months from re-irradiation, with shorter median follow-up times than the studies included, with fatal tracheoesophageal fistulae in 30% of patients (10). Another re-irradiation experience for EC (all SCC), using IMRT (56%) or 3D-CRT (44%), found that at a median follow-up of 87 months, 90% of patients experienced treatment failure, with 9%, 15%, and 24% rates of tracheoesophageal fistulae, pericardial/pleural effusion, and RT pneumonitis, respectively (47). Thus, PBT re-irradiation for EC compares favorably with published non-PBT experiences, potentially taking advantage of the physical properties of protons to spare dose to OARs and reduce toxicity, and is a viable option for patients with EC.
In PC, PBT re-irradiation provided reasonable LC (73%) and survival (median OS 17 months) at a median follow-up of 15.7 months, delivered concurrently with chemotherapy in most patients, with acceptable toxicity (13% acute grade 3, 7% acute grade 4, 7% acute grade 5 favored to be due to disease progression rather than PBT, and no late grade ≥2 toxicities) (42). These results compare favorably with published experiences of SBRT re-irradiation for PC, in which 1-year LC and median OS ranged from 62–78% and 9–14 months, respectively, with acute grade ≥3 and late grade ≥3 toxicity rates ranging from 0–10% and 6–7% (for SBO), respectively (13,14). While it is difficult to comment directly on the dosimetric differences between the PBT plans and the SBRT plans, it is possible that PBT’s rapid dose fall-off may have spared otherwise unavoidable bowel dose, while achieving comparable disease-control.
The data for PBT re-irradiation in recurrent or second primary liver tumors, while heterogeneous, demonstrate reasonable disease control (1-year LR 46%) and survival (median OS 14-61 months, with 5-year OS 49%), with excellent tolerability of treatment (2 acute grade ≥3 toxicities across both studies, no late grade ≥3 toxicities, and no cases of RILD) (43,44). The tolerability of effective therapy is particularly important in this patient population, as many have impaired baseline liver function, significant medical comorbidities, and exhausted alternative treatment options. In the studies referenced, patients were re-irradiated with PBT at least once, often for multiple liver tumors, with a variety of primary cancers (HCC, IHCC, and metastases), many prior non-RT treatments, and nearly one-third of patients with Child-Pugh B-C liver function in Oshiro et al. Thus, while the studies’ heterogeneity makes direct comparisons to other cohorts difficult, it also speaks to the breadth of scenarios in which PBT re-irradiation can be an effective option for patients with liver tumors. Further, prior studies of photon re-irradiation for HCC reported 36% and 25% rates of RILD and treatment-related death, respectively, underscoring the promise of PBT in this setting (48).
In RC, PBT re-irradiation also provided reasonable disease control and survival, with acceptable toxicity (4 acute grade 3, no acute grade ≥4 and 3 late grade 4 toxicities), at a median follow-up of 19.4 months (45). Two of the late grade 4 toxicities (SBO and entero-vaginal fistula) occurred in the same patient. The SBO occurred in the post-operative period, following PBT, and the fistula was felt to be due to tumor progression. This small study compares well with multiple prior reports of photon re-irradiation for RC. Sun et al. (11) found that hyperfractionated 3D-CRT re-irradiation with concurrent capecitabine for LR RC had an overall response rate of 60%, median OS of 32 months, 10% and 8% acute grade 3–4 diarrhea and granulocytopenia, respectively, and 1.4% rate of severe late toxicity (SBO), at a median follow-up of 24 months. Youssef et al. (12) found that IMRT re-irradiation with concurrent chemotherapy (81%) for LR RC had a 2-year LR rate of 53%, median OS of 22 months, 3% acute grade ≥3 toxicity (diarrhea), and 3% late grade ≥3 (sacral insufficiency fracture), at a median follow-up of 11.3 months. In addition, the results for PBT re-irradiation are comparable to that of carbon ion re-irradiation for LR RC, where Habermehl et al. (16) found that at a median follow-up of 7.8 months, the 1-year LC rate was 79% without grade ≥3 toxicities. Given the difference between the median follow-up intervals of the PBT and CIT studies, it is difficult to make a meaningful comparison at present.
In AC, our discussion is limited beyond treatment-planning aspects, as the sole published report of PBT re-irradiation for AC at this time did not report disease-control, survival, or toxicity outcomes (46). Additional studies in this scenario would be of value.
While PBT re-irradiation for GI malignancies was well-tolerated overall, there were instances of substantial toxicity felt to be RT-related, which physicians should consider carefully. In Boimel et al.’s (42) report of PBT re-irradiation for PC, 1 patient developed an acute grade 4 bleeding duodenal ulcer. This patient previously underwent biliary stent placement 9 months before PBT re-irradiation, then received PBT to the primary tumor, which contained duodenum within the PBT field. One week after completion of PBT, the ulcer was discovered and treated. One patient in this study suffered gastric outlet obstruction due to disease progression at the end of PBT re-irradiation, and subsequently required stent placement 3 days after PBT completion, complicated by fatal small bowel perforation, favored to be due to progressive disease and instrumentation, but potentially related to RT. These cases highlight the importance of exercising caution when delivering RT near the duodenum, an organ that commonly exhibits RT toxicity. Additionally, a prior dosimetric comparison of PBT and IMRT for unresectable PC found that while PBT decreased duodenal dose in low-dose regions, it increased duodenal dose in the mid-high-dose regions (49). Thus, even with the most conformal techniques, including PBT, significant and fatal duodenal toxicity can still occur, particularly in the setting of progressive disease or instrumentation, and these should be considerations when deciding whether or not to offer re-irradiation near the duodenum.
Two studies included generated comparison VMAT/IMRT plans to analyze dosimetric differences from the treated PBT plans. In Patel et al.’s (41) report, PBT reduced mean heart and lung doses, spinal cord maximum dose, and lung V5Gy for all patients, as compared to VMAT, while lung V20Gy and V30Gy were higher for PBT in the 2 patients treated with PS. Berman et al. (45) found that PBT significantly decreased bowel V10Gy and V20Gy, and the dose to 200 and 150 cc of bowel, as compared to IMRT. These are consistent with Thompson et al.’s (49) findings that PBT significantly reduced stomach, duodenal and small bowel dose in low-dose regions, as compared to IMRT. While the toxicity results in the studies included are encouraging, it still remains to be seen in a prospective, controlled, comparative setting whether these dosimetric advantages for PBT will translate into clinical advantages over alternative RT modalities.
There are a number of limitations to this publication. It is possible that additional PBT re-irradiation literature for GI malignancies exists, either in non-English languages, or outside of the PubMed search engine. Another limitation is that nearly all PBT treatments delivered across each of the studies were delivered with techniques other than PBS. It is possible, as evidenced by Patel et al.’s (41) case series, that PBS may provide reduction of OAR doses beyond that of PS, which could translate into further clinical benefit. If so, the results in this analysis may overestimate PBT toxicity or underestimate a potential benefit. A specific limitation within McDuff et al.’s (44) report is that 90% of patients did not receive PBT, and the majority of outcomes reported did not stratify into PBT versus non-PBT modalities. While it was specified that neither of the RILD cases occurred in PBT patients, beyond the overwhelmingly positive toxicity outcomes for the entire cohort, it is difficult to draw more meaningful conclusions without further specification. Many of the reports included in this analysis were single-arm, retrospective studies of PBT, without discrete, randomized, controlled, or propensity-matched comparisons to non-PBT modalities. Thus, any comparisons between the PBT studies included and other re-irradiation studies must be interpreted with caution. Further prospective, randomized comparisons between PBT and standard modalities, as well as other advanced modalities, such as CIT, can help provide additional clarity. Some of the studies included had short median follow-up intervals, and may not have captured additional outcomes that occurred with longer follow-up. To better assess PBT’s role in the re-irradiation setting for GI malignancies, longer-term follow-up is warranted.
Studies were assessed for bias, as in Verma et al.’s (38) review of PBT re-irradiation, by considering sample size, study design, consistency of presented results, patient eligibility/selection, outcome measures, follow-up intervals, potential for selective reporting/publication bias, external validity, funding bias, and discussion of limitations. Many studies included in our analysis were limited by small sample size, retrospective single-arm design, differing definitions of outcomes, incomplete statements of patient eligibility/selection, short follow-up, potential for selective reporting/publication bias, and limited external validity. Funding bias was not observed. Study limitations were discussed, to varying degrees. In general, there is risk of bias within the studies, and the evidence quality is limited by the above issues.
Based upon the published experiences to date, PBT re-irradiation for recurrent or second primary GI malignancies appears to be an effective option for palliation, with encouraging disease-control, survival, and toxicity outcomes, favorable dosimetric comparisons to standard RT modalities, and compares well with published non-PBT GI re-irradiation experiences. In our own experience, we have learned that re-irradiation with PBT is extremely resource intensive, from obtaining old records and creating plan sums, to debate in tumor boards to help select patients. The importance of careful patient selection cannot be overstated. In general, we attempt to limit PBT re-irradiation to patients with good performance statuses, more than 1 year from prior RT, and reasonable target volumes, with careful attention to OAR doses in past and re-irradiation courses. Of particular note is the risk of stents and tubes within hollow viscous organs getting high cumulative RT doses. It is not known if these mechanical stressors increase toxicity risk, but several of the severe PBT re-irradiation toxicities occurred in the setting of iatrogenic microtraumas. Ultimately, the complicated decision to re-irradiate is colored by the balance between the known (often 100%) risk of tumor-associated complications compared to the unknown risk of re-irradiation toxicities, where there are no other therapeutic options. Given the novelty of PBT and short follow-up in the published studies, additional study is warranted to determine if the dosimetric advantages seen in PBT will translate into short- and long-term toxicity benefits.
Acknowledgments
None.
Footnote
Conflicts of Interest: JM Metz is on the advisory board of Varian Medical Systems, Ion Beam Applications, and Provision. JP Plastaras serves on committees for the American Board of Radiology, the American Society for Radiation Oncology, and the International Lymphoma Radiation Oncology Group. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- van Hagen P, Hulshof MCCM, van Lanschot JJB, et al. Preoperative Chemoradiotherapy for Esophageal or Junctional Cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after Surgery Compared with Surgery Alone for Adenocarcinoma of the Stomach or Gastroesophageal Junction. N Engl J Med 2001;345:725-30. [Crossref] [PubMed]
- Gastrointestinal Tumour Study Group. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Cancer 1987;59:2006-10. [Crossref] [PubMed]
- Loehrer PJ, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: An Eastern Cooperative Oncology Group trial. J Clin Oncol 2011;29:4105-12. [Crossref] [PubMed]
- Ben-Josef E, Guthrie KA, El-Khoueiry AB, et al. SWOG S0809: A phase II intergroup trial of adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine in extrahepatic cholangiocarcinoma and gallbladder carcinoma. J Clin Oncol 2015;33:2617-22. [Crossref] [PubMed]
- Hong TS, Wo JY, Yeap BY, et al. Multi-institutional phase II study of high-dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol 2016;34:460-8. [Crossref] [PubMed]
- Peeters KCMJ, Marijnen CAM, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: Increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg 2007;246:693-701. [Crossref] [PubMed]
- Nigro ND, Vaitkevicius VK, Buroker T, et al. Combined therapy for cancer of the anal canal. Dis Colon Rectum 1981;24:73-5. [Crossref] [PubMed]
- Janot F, de Raucourt D, Benhamou E, et al. Randomized Trial of Postoperative Reirradiation Combined With Chemotherapy After Salvage Surgery Compared With Salvage Surgery Alone in Head and Neck Carcinoma. J Clin Oncol 2008;26:5518-23. [Crossref] [PubMed]
- Kim YS, Lee CG, Kim KH, et al. Re-irradiation of recurrent esophageal cancer after primary definitive radiotherapy. Radiat Oncol J 2012;30:182-8. [Crossref] [PubMed]
- Sun DS, Zhang JD, Li L, et al. Accelerated hyperfractionation field-involved re-irradiation combined with concurrent capecitabine chemotherapy for locally recurrent and irresectable rectal cancer. Br J Radiol 2012;85:259-64. [Crossref] [PubMed]
- Youssef FF, Parikh PJ, DeWees TA, et al. Efficacy and toxicity of rectal cancer reirradiation using IMRT for patients who have received prior pelvic radiation therapy. Adv Radiat Oncol 2016;1:94-100. [Crossref] [PubMed]
- Dagoglu N, Callery M, Moser J, et al. Stereotactic Body Radiotherapy (SBRT) Reirradiation for Recurrent Pancreas Cancer. J Cancer 2016;7:283-8. [Crossref] [PubMed]
- Wild AT, Hiniker SM, Chang DT, et al. Re-irradiation with stereotactic body radiation therapy as a novel treatment option for isolated local recurrence of pancreatic cancer after multimodality therapy: Experience from two institutions. J Gastrointest Oncol 2013;4:343-51. [PubMed]
- Shirai K, Ohno T, Saitoh J, et al. Prospective Study of Isolated Recurrent Tumor Re-irradiation With Carbon-Ion Beams. Front Oncol 2019;9:181. [Crossref] [PubMed]
- Habermehl D, Wagner M, Ellerbrock M, et al. Reirradiation Using Carbon Ions in Patients with Locally Recurrent Rectal Cancer at HIT: First Results. Ann Surg Oncol 2015;22:2068-74. [Crossref] [PubMed]
- Patyal B. Dosimetry Aspects of Proton Therapy. Technol Cancer Res Treat 2007;6:17-23. [Crossref] [PubMed]
- Ryckman JM, Ganesan V, Kusi Appiah A, et al. National practice patterns of proton versus photon therapy in the treatment of adult patients with primary brain tumors in the United States. Acta Oncol 2019;58:66-73. [Crossref] [PubMed]
- Gunn GB, Blanchard P, Garden AS, et al. Clinical Outcomes and Patterns of Disease Recurrence after Intensity Modulated Proton Therapy for Oropharyngeal Squamous Carcinoma. Int J Radiat Oncol Biol Phys 2016;95:360-7. [Crossref] [PubMed]
- Lewis GD, Holliday EB, Kocak-Uzel E, et al. Intensity-modulated proton therapy for nasopharyngeal carcinoma: Decreased radiation dose to normal structures and encouraging clinical outcomes. Head Neck 2016;38 Suppl 1:E1886-95. [Crossref] [PubMed]
- Vogel J, Berman AT, Lin L, et al. Prospective study of proton beam radiation therapy for adjuvant and definitive treatment of thymoma and thymic carcinoma: Early response and toxicity assessment. Radiother Oncol 2016;118:504-9. [Crossref] [PubMed]
- Remick JS, Schonewolf C, Gabriel P, et al. First Clinical Report of Proton Beam Therapy for Postoperative Radiotherapy for Non-Small-Cell Lung Cancer. Clin Lung Cancer 2017;18:364-71. [Crossref] [PubMed]
- Rwigema JM, Verma V, Lin L, et al. Prospective study of proton-beam radiation therapy for limited-stage small cell lung cancer. Cancer 2017;123:4244-51. [Crossref] [PubMed]
- Verma V, Shah C, Mehta MP. Clinical Outcomes and Toxicity of Proton Radiotherapy for Breast Cancer. Clin Breast Cancer 2016;16:145-54. [Crossref] [PubMed]
- Verma V, Iftekaruddin Z, Badar N, et al. Proton beam radiotherapy as part of comprehensive regional nodal irradiation for locally advanced breast cancer. Radiother Oncol 2017;123:294-8. [Crossref] [PubMed]
- Verma V, Simone CB, Wahl AO, et al. Proton radiotherapy for gynecologic neoplasms. Acta Oncol 2016;55:1257-65. [Crossref] [PubMed]
- Deville C, Jain A, Hwang WT, et al. Initial report of the genitourinary and gastrointestinal toxicity of post-prostatectomy proton therapy for prostate cancer patients undergoing adjuvant or salvage radiotherapy. Acta Oncol 2018;57:1506-14. [Crossref] [PubMed]
- Fang P, Mick R, Deville C, et al. A case-matched study of toxicity outcomes after proton therapy and intensity-modulated radiation therapy for prostate cancer. Cancer 2015;121:1118-27. [Crossref] [PubMed]
- Badiyan SN, Hallemeier CL, Lin SH, et al. Proton beam therapy for gastrointestinal cancers: past, present, and future. J Gastrointest Oncol 2018;9:962-71. [Crossref] [PubMed]
- Mizumoto M, Okumura T, Ishikawa E, et al. Reirradiation for recurrent malignant brain tumor with radiotherapy or proton beam therapy. Technical considerations based on experience at a single institution. Strahlenther Onkol 2013;189:656-63. [Crossref] [PubMed]
- Galle JO, McDonald MW, Simoneaux V, et al. Reirradiation with Proton Therapy for Recurrent Gliomas. Int J Part Ther 2015;2:11-8. [Crossref]
- Romesser PB, Cahlon O, Scher ED, et al. Proton Beam Reirradiation for Recurrent Head and Neck Cancer: Multi-institutional Report on Feasibility and Early Outcomes. Int J Radiat Oncol Biol Phys 2016;95:386-95. [Crossref] [PubMed]
- McDonald MW, Zolali-Meybodi O, Lehnert SJ, et al. Reirradiation of Recurrent and Second Primary Head and Neck Cancer With Proton Therapy. Int J Radiat Oncol Biol Phys 2016;96:808-19. [Crossref] [PubMed]
- McAvoy SA, Ciura KT, Rineer JM, et al. Feasibility of proton beam therapy for reirradiation of locoregionally recurrent non-small cell lung cancer. Radiother Oncol 2013;109:38-44. [Crossref] [PubMed]
- Chao HH, Berman AT, Simone CB, et al. Multi-Institutional Prospective Study of Reirradiation with Proton Beam Radiotherapy for Locoregionally Recurrent Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:281-92. [Crossref] [PubMed]
- Thorpe CS, Niska JR, Girardo ME, et al. Proton beam therapy reirradiation for breast cancer: Multi-institutional prospective PCG registry analysis. Breast J 2019;25:1160-70. [Crossref] [PubMed]
- Li YR, Kirk M, Lin L. Proton Therapy for Vaginal Reirradiation. Int J Part Ther 2016;3:320-6. [Crossref] [PubMed]
- Verma V, Rwigema JCM, Malyapa RS, et al. Systematic assessment of clinical outcomes and toxicities of proton radiotherapy for reirradiation. Radiother Oncol 2017;125:21-30. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Fernandes A, Berman AT, Mick R, et al. A Prospective Study of Proton Beam Reirradiation for Esophageal Cancer. Int J Radiat Oncol Biol Phys 2016;95:483-7. [Crossref] [PubMed]
- Patel SA, Edgington SK, Adams J, et al. Novel use of proton beam therapy for neoadjuvant treatment of radiation-associated squamous cell carcinoma of the esophagus. J Gastrointest Oncol 2019;10:155-60. [Crossref] [PubMed]
- Boimel PJ, Berman AT, Li J, et al. Proton beam reirradiation for locally recurrent pancreatic adenocarcinoma. J Gastrointest Oncol 2017;8:665-74. [Crossref] [PubMed]
- Oshiro Y, Mizumoto M, Okumura T, et al. Analysis of repeated proton beam therapy for patients with hepatocellular carcinoma. Radiother Oncol 2017;123:240-5. [Crossref] [PubMed]
- McDuff SGR, Remillard KA, Zheng H, et al. Liver reirradiation for patients with hepatocellular carcinoma and liver metastasis. Pract Radiat Oncol 2018;8:414-21. [Crossref] [PubMed]
- Berman AT, Both S, Sharkoski T, et al. Proton Reirradiation of Recurrent Rectal Cancer: Dosimetric Comparison, Toxicities, and Preliminary Outcomes. Int J Part Ther 2014;1:2-13. [Crossref]
- Apinorasethkul O, Lenards N, Hunzeker A. Urethral dose sparing in squamous cell carcinoma of anal canal using proton therapy matching electrons with prior brachytherapy for prostate cancer: A case study. Med Dosim 2016;41:242-7. [Crossref] [PubMed]
- Hong L, Huang YX, Zhuang QY, et al. Survival benefit of re-irradiation in esophageal Cancer patients with Locoregional recurrence: a propensity score-matched analysis. Radiat Oncol 2018;13:171. [Crossref] [PubMed]
- Huang Y, Chen SW, Fan CC, et al. Clinical parameters for predicting radiation-induced liver disease after intrahepatic reirradiation for hepatocellular carcinoma. Radiat Oncol 2016;11:89. [Crossref] [PubMed]
- Thompson RF, Mayekar SU, Zhai H, et al. A dosimetric comparison of proton and photon therapy in unresectable cancers of the head of pancreas. Med Phys 2014;41:081711. [Crossref] [PubMed]


