Exploring signet-ring cells in pregnant female
Introduction
Primary signet-ring cell carcinoma (SRCC) of the colon and rectum is a rare form of adenocarcinoma of the large intestine and is responsible for less than 0.4% of all fatal malignancies. The incidence of diagnosing a cancer of the colon during pregnancy is estimated to be less than 0.1% (1). Patients with SRCC of the colon and rectum are young and usually diagnosed at an advanced stage, have an aggressive clinical behaviour, variable growth patterns and poor average survival and it is difficult to comment on their effective treatment strategy. Here, we are presenting a young pregnant female with advanced SRCC and exploring the different possibilities of management and response evaluation.
Case report
A 26-year-old young female presented with constipation and blood in stools with mild-pain during defecation. Patient was non-alcoholic, non-smoker with a history of significant sun exposure throughout her life. She also had a history of weight-loss. General physical and systemic examination was normal. Complete hemogram and routine blood biochemistry parameters of the patient were within normal limits. A chest radiograph did not indicate any metastatic nodules. Trans urethral sonography (TUSG) abdomen showed single live intrauterine fetus of 10-week gestation and rectal thickening. On per rectal examination, a tender circumferential stricture was present 2-cm above the anal verge.
Histological examination
A biopsy was taken from the growth anorectum, which revealed poorly differentiated adenocarcinoma with signet ring morphology with wide spread positivity for cytokeratin & p53 (Figure 1).
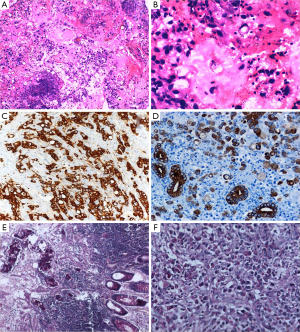
The histological appearance of the tumor is characterized by cells with abundant intracytoplasmic mucin, which pushes the nucleus to the periphery (Figure 1B). The tumor cells may be sequenced individually or in clusters and may diffusely spread through the bowel wall. In general, SRCC shows the characteristic appearance of “linitis plastica” and behaves more aggressively than carcinoma of other histological types (2-4).
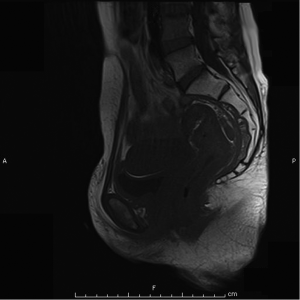
Magnetic resonant imaging (MRI) pelvis showed thickening involving anorectum 1.2 cm from the anal verge and extending over 4.2 cm cranio-caudally. It was abutting the right puborectalis muscle. Uterus was anteverted and showed gestational sac with fetus in situ. In view of the disease as seen on MRI pelvis (Figures 2-4), she underwent medical termination of pregnancy (MTP). Then, she reported 2-months later with a repeat MRI scan of pelvis, which revealed soft tissue lesion involving anorectum and growth extending over 10-cm cranio-caudally with involvement of perirectal fat, mesorectal fascia and reaching up to the right lateral pelvic wall. Posterior paravaginal fat planes were effaced with thickening which was abutting the posterior vaginal wall. Planes with bilateral adnexa effaced as compared to previous study. Moderate to marked progression in disease was present. Enlarged perirectal nodes of size 1.3 cm were present. With this diagnosis of advanced signet ring adenocarcinoma anorectum, the patient received external beam radiotherapy on LINAC with 9 Gy/5#/5-days followed by concomitant chemoradiation with external beam radiotherapy using IMRT with a D95 of 41.76 Gy in 23# along with injection 5-fluorouracil (5-FU) 1,050 mg day 1 to 4. After the completion of chemoradiation, patient underwent colostomy, following which the MRI abdomen and pelvis revealed residual disease in form of thickening involving the anal verge and anal canal with mild to moderate regression from previous study and increased retroperitoneal nodes (Figure 5). She further underwent 6-cycles of 2-weekly intravenous adjuvant chemotherapy with FOLFOX-4 regimen with Inj Oxaliplatin 85 mg/m2 day 1; Inj Leucovorin 200 mg/m2, days 1 and 2 before 5-FU; and 5-FU 400 mg/m2 IV bolus on day 1 then 5-FU 600 mg/m2 as a 22-hour infusion given on days 1 and 2 every 14 days. She further underwent colonoscopy which revealed residual disease, after which she received 5-more courses of FOLFOX-4 for 2½ months. Subsequently, patient was lost to follow up after 3-month of completion of chemotherapy.
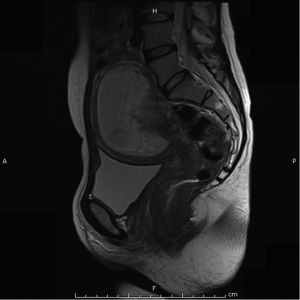
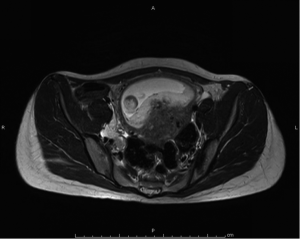
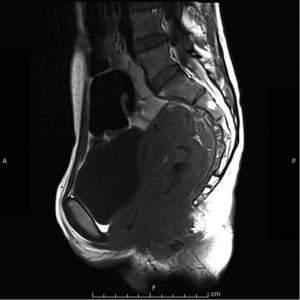
Discussion
Since its proposal in 1951, Laufman and Saphir, first described primary SRCC, a rare variant of colorectal adenocarcinomas (3). The incidence of SRCC is reported to range between 0.1-0.9% (4). According to a study by Secco et al. conducted in 1994, the 5-year survival rate of primary colorectal SRCC was zero (median 15 months) and disease recurrence was 100% (5). However, a study from Singapore reported 5-year survival rate of 12% showing a little variation with the former study (6). It is a rare but distinct malignancy of the large bowel. More than 96% of cases of SRCC arise in the stomach and the rest occur in the colon, rectum, gall bladder, pancreas, urinary bladder and breast, which further explains the rarity of the present case (2,7).
SRCC have been analyzed for microsatellite instability, which is present in approximately 30% of tumors (8). Furthermore, mutations of K-ras and p53 gene have been reported in SRCC of the colon and rectum; however, the frequency of K-ras gene mutation in SRCC is considerably lower than that of moderately and well differentiated carcinomas (9). Replications of DNA are suggested to be at least partly involved in the carcinogenesis of SRCC.
Colorectal cancer is often insidious in development, and fatigue, anaemia, altered bowel function and weight losses are frequent symptoms. The most common acute surgical problem reported is intestinal obstruction, which is reported in about 30% of left-sided lesions as in our present case. The diagnosis of colon cancer is best established by colonoscopy, which provides direct visualization, a fairly accurate determination of location, and the opportunity to obtain tumor tissue for histologic evaluation. Intraoperative ultrasound is the most sensitive way to evaluate the liver. The liver is the most common site for synchronous metastases. Tumor size is not as critical as are depth of invasion and nodal status in determining prognosis. High histologic grade, lymphatic invasion, venous invasion and involvement of surgical resection margins are independent adverse prognostic factors (10). However, careful rectal examination yields 67-84% accuracy in staging of rectal carcinomas. MRI with endorectal coil is equivalent to ultrasound and both are more sensitive and accurate and are used to assess locally advanced or recurrent local disease (11).
In colorectal adenocarcinoma, surgery is the main stay of treatment. The extent of resection is determined by tumor size, location, histologic grade and tumor extension into the colon wall and into adjacent tissue or organs (10). Adjuvant chemotherapy is considered in stage II with a high risk of recurrence and stage III. In stage IV disease, palliative management should be preferred and emphasis should be to lengthen the progression-free and overall survival in unresectable metastatic colorectal cancers (11). Adjuvant radiotherapy is indicated in patients with close or positive surgical margins or in patients with T4 lesions adherent to the pelvic structure. Initial dose of radiation treatment is administered through parallel opposed or other multi-field techniques to treat the tumor bed with an approximate 3 to 5-cm margin to a total dose of 45 Gy, followed by reduced fields to a total dose of 50.4 to 54 Gy. Concomitant chemoradiation is indicated for tumor invading adjacent structures, those complicated by perforation and fistula or incomplete resection. Neoadjuvant chemoradiation attempting for sphincter sparing for mid to low rectal cancer should be discussed with patients. Patients with T3, tethered, or poorly differentiated tumors are frequently treated with higher dose of pre-operative irradiation (45-46 Gy), frequently combined with chemotherapy (5-FU) and later followed by surgery (12).
The incidence of diagnosing a cancer of the colon during pregnancy is estimated to be less than 0.1%. It is interesting that pregnant women have a much higher incidence of rectal cancer (83%), compared with colon sites (17%). This is in contrast to non-pregnant women younger than 40-years of age, among whom the site distribution is 68% colon and 32% rectum respectively (10). Certainly, any pregnant patient who reports rectal bleeding or has hemoccult positive stool on examination deserves careful evaluation to rule out cancer. Colonoscopy in pregnancy is a relative contraindication and the patient should be fully informed of the possible maternal and fetal risks (13). Measures used to improve safety of endoscopy in pregnant patients include gentle abdominal compression during colonic intubation and the use of meperidine because of its safer fetal profile (14). Also, oxygen should be administered to the pregnant patients during endoscopy to decrease potential risks of hypoxia from sedation (15). Serum CEA levels during pregnancy are usually normal but may be slightly elevated. Abdominal CT imaging is contraindicated in pregnancy due to radiation teratogenicity, particularly in the first trimester (16). An abdominal ultrasound is a good alternate to abdominal CT imaging; this is useful in evaluating the presence of hepatic metastasis and has a sensitivity of 75% for detecting macro-metastatic lesions (17). Resection of the colorectal cancer at the time of delivery can be considered if a caesarean section is performed.
The complex treatment of colorectal cancer in pregnancy is based on the gestational age of the fetus, tumor stage and need for emergent vs. elective surgery. If the diagnosis occurs in the first half of pregnancy as in our present case (at less than 20 weeks), significant tumor progression may occur if surgery is delayed until fetus is viable; thus the clinician should recommend MTP and proceed with resection of the tumor. If the diagnosis is made in the second half of pregnancy (more than 20 weeks), resection could be delayed until the infant is delivered. Following the birth, the patient can be treated in the same manner as a non-pregnant patient. Incidence of ovarian metastases from colorectal tumors is 25% higher in pregnant patients. As in our present case report, SRCC of the colon and rectum are usually diagnosed at an advanced stage, because symptoms usually develop late. Thereafter, cancers limited to the mucosal and sub-mucosal layers are rarely detected.
Conclusions
Primary SRCC of the colon and rectum in pregnant female is a rare form of adenocarcinoma of the large intestine. SRCC of the colon and rectum are usually diagnosed at an advanced stage and are usually younger, more likely to experience lymph node metastasis, have an aggressive clinical course and poor prognosis. Diagnosis of a colon primary is supported by the presence of abundant intracytoplasmic mucin with wide spread positivity for cytokeratin. Surgery is the main stay of treatment while in adjuvant and neoadjuvant settings, RT and CRT have significant role in accordance to the site of tumor. Measures used to improve safety of endoscopy include use of meperidine and oxygen to decrease the potential risks of hypoxia. Abdominal CT is contraindicated in pregnancy due to radiation teratogenicity. USG and MR scan is an alternate to CT imaging. If the diagnosis occurs in the first half of pregnancy, termination of pregnancy is recommended and proceeds with resection of the tumor. If the diagnosis is made in the second half, resection could be delayed until the infant is delivered.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kim JH, Park SJ, Park MI, et al. Early-stage primary signet ring cell carcinoma of the colon. World J Gastroenterol 2013;19:3895-8. [PubMed]
- Sim HL, Tan KY, Poon PL, et al. Primary rectal signet ring cell carcinoma with peritoneal dissemination and gastric secondaries. World J Gastroenterol 2008;14:2118-20. [PubMed]
- Laufman H, Saphir O. Primary linitis plastica type of carcinoma of the colon. AMA Arch Surg 1951;62:79-91. [PubMed]
- Almagro UA. Primary signet-ring carcinoma of the colon. Cancer 1983;52:1453-7. [PubMed]
- Secco GB, Fardelli R, Campora E, et al. Primary mucinous adenocarcinomas and signet-ring cell carcinomas of colon and rectum. Oncology 1994;51:30-4. [PubMed]
- Ooi BS, Ho YH, Eu KW, et al. Primary colorectal signet-ring cell carcinoma in Singapore. ANZ J Surg 2001;71:703-6. [PubMed]
- Urabe T, Kuroda Y, Urushihara T, et al. Two-mm diameter signet ring cell carcinoma of the rectum with lymph node metastasis: a report of case. Stomach Intest 1998;33:1179-83.
- Seidman DS, Heyman Z, Ben-Ari GY, et al. Use of magnetic resonance imaging in pregnancy to diagnose intussusception induced by colonic cancer. Obstet Gynecol 1992;79:822-3. [PubMed]
- Kawabata Y, Tomita N, Monden T, et al. Molecular characteristics of poorly differentiated adenocarcinoma and signet-ring-cell carcinoma of colorectum. Int J Cancer 1999;84:33-8. [PubMed]
- Compton C, Hawk E, Grochow L, et al. Colon cancer. In: Abeloff M, Armitage J, Niederhuber J, et al. eds. Abeloff’s Clinical Oncology. 4th ed. Philadelphia: Churchill Livingstone, 2008:1477-525.
- Cohen A, Garofalo MC, DeSimone PA, et al. Cancer of the rectum. In: Abeloff M, Armitage J, Niederhuber J, et al. eds. Abeloff’s Clinical Oncology. 4th ed. Philadelphia: Churchill Livingstone, 2008:1535-52.
- Chao KS, Perez CA, Brady LW. Colon and rectum. In: Chao KS, Perez CA, Brady LW. eds. Radiation Oncology: Management Decisions. 3rd ed. Philadelphia: Lippincott Williams and Wilkins, 2011:448.
- Bernstein MA, Madoff RD, Caushaj PF. Colon and rectal cancer in pregnancy. Dis Colon Rectum 1993;36:172-8. [PubMed]
- Cappell MS. Colon cancer during pregnancy. The gastroenterologist’s perspective. Gastroenterol Clin North Am 1998;27:225-56. [PubMed]
- Dark DS, Campbell DR, Wesselius LJ. Arterial oxygen desaturation during gastrointestinal endoscopy. Am J Gastroenterol 1990;85:1317-21. [PubMed]
- Brent RL. The effect of embryonic and fetal exposure to x-ray, microwaves, and ultrasound: counseling the pregnant and nonpregnant patient about these risks. Semin Oncol 1989;16:347-68. [PubMed]
- Nies C, Leppek R, Sitter H, et al. Prospective evaluation of different diagnostic techniques for the detection of liver metastases at the time of primary resection of colorectal carcinoma. Eur J Surg 1996;162:811-6. [PubMed]


