
An unusual case of primary acinar cell carcinoma of the liver and its treatment
Introduction
Acinar cell carcinoma (ACC) is an epithelial neoplasm characterized by morphological features similar to acinar cells found in exocrine glands (1). Per literature review, ACC has been reported in the pancreas, salivary glands, prostate gland and lungs (2). Regardless of where the tumor originates, the majority of acinar neoplasms are found to be solid and malignant (1). Rare benign cystic neoplasms of the pancreas have been reported and are referred to as acinar cell cystadenomas (1). Many cases of ACCs are diagnosed in the pancreas and make up approximately 1–2% of total pancreatic malignancies (3,4). Compared to ductal carcinomas, ACCs of the pancreas are associated with better prognosis, increased survival, and less aggressive behavior (1,4). Due to the rarity of these malignancies, no standardized treatment protocols have been developed thus far (4). Most hepatic ACCs have evidence of pancreatic exocrine enzyme production, and majority of those cases are metastases from primary pancreatic tumors (3,5). This could be possibly due to the liver’s close proximity to the pancreas. However, the number of cases reporting hepatic ACCs as the primary malignancy instead of a metastatic tumor have been limited. The pancreas in each of those patients in the reported cases was found to be without lesions, and no other sources of cancers were detected. One of the hypotheses suggesting hepatic ACC as a primary malignancy proposes the idea of an ectopic origin of acinar cells in the liver, which is thought to eventually undergo malignant transformation (6). In this report we present such a case, where the patient was found to have a primary hepatic ACC with the absence of a pancreatic tumor.
Case presentation (Figures 1-4)
The patient is a 59-year-old female with no significant past medical history who was residing in Mexico when she became symptomatic with severe abdominal pain. Initial workup by her primary care physician included a computed tomography (CT) scan of her abdomen which revealed hepatomegaly and multiple mass lesions. A biopsy of the hepatic lesion was obtained, revealing adenocarcinoma of an unknown primary source. The patient subsequently underwent a colonoscopy which was negative for any signs of malignancy.
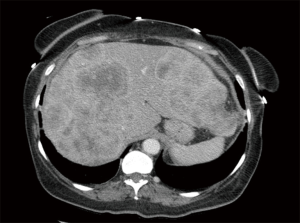
After the initial workup in Mexico, the patient traveled to the United States where she continued to have severe abdominal pain, decreased appetite and early satiety. She was then referred to medical oncology for further evaluation. Physical examination during the initial oncology visit revealed a markedly enlarged and tender liver, extending 9–10 cm below the right costal margin, and 8–9 cm below the xiphoid process. Her vital signs were stable, and laboratory studies were only significant for elevated alkaline phosphatase 340 U/L, aspartate aminotransferase (AST) 61 U/L, total bilirubin 2.0 mg/dL, and direct bilirubin 1.2 mg/dL. A CT scan of the abdomen and pelvis with contrast showed innumerable multifocal heterogeneously enhancing mass lesions throughout the liver, concerning for metastatic disease (Figure 1). The pancreas appeared unremarkable without any mass lesions. Tumor markers were obtained at that time and were remarkable for an elevated carcinoembryonic antigen (CEA) at 5,920 ng/mL and CA125 at 80.7 U/mL, respectively. CA19-9, alpha-fetoprotein and CA27-29 were negative. She had no other complaints and no swelling or tenderness in her jaw concerning for parotid or salivary gland pathology. Repeat biopsy of a lesion in the left hepatic lobe showed reactive hepatocyte abnormalities with mild acute and chronic inflammation.
The patient’s symptoms continued to worsen and she was admitted to the hospital for pain management. A repeat CT of the abdomen and pelvis again demonstrated innumerable multifocal heterogeneous mass lesions throughout the liver, similar to the previous scan. Repeat labs were consistent with prior findings of elevated alkaline phosphatase, AST, and bilirubin.
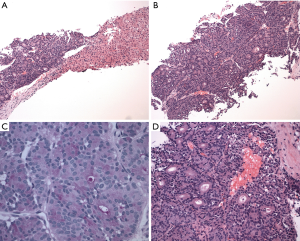
During this admission, a biopsy was obtained from the right liver mass which demonstrated well to moderately differentiated adenocarcinoma and foci of necrosis, suggestive of ACC. The tumor consisted of a cellular neoplasm arranged in nested, glandular and acinar patterns (Figure 2A). No desmoplasia was identified (Figure 2D). The lesional cells were composed of monotonous cells with basal nuclei, moderate nuclear atypia, prominent nucleoli and abundant eosinophilic granular apical cytoplasm (Figure 2B). There was variable mitosis noted.
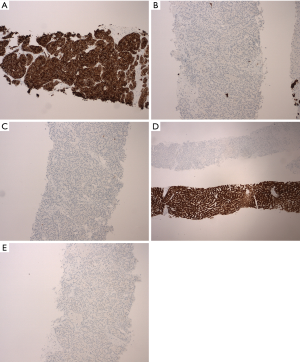
Immunohistochemical staining (Figure 2A,B,C,D and Figure 3A,B,C,D,E) was performed for further characterization. The tumor cells were positive for high molecular weight (HMW) keratin (Figure 3A), and the Periodic acid-Schiff (PAS) and PAS with diastase highlighted cytoplasmic granules (Figure 2C). The tumor cells were negative for cytokeratin 7 (CK 7) (Figure 3B), CK 20 (Figure 3C), arginase (Figure 3D), CD56 (Figure 3E), thyroid transcription factor 1 (TTF-1), estrogen receptor (ER) and GATA binding protein 3 (GATA-3). HMW keratins are expressed in squamous and transitional epithelium, basal cells, and ductal cells (7). PAS is commonly used to stain glycogen and mucin (8); diastase is employed to differentiate glycogen from mucin—glycogen is sensitive to digestion, but mucin is resistant (8). CK are cytoskeletal proteins present in epithelial cells and can be classified according to molecular weight and acidity/basicity (8). CK 7 and CK 20 have organ specific staining patterns and are commonly used to identify carcinomas of unknown primary origin (8). Arginase is a marker for hepatocellular differentiation (8). CD56 is normally expressed on neuroendocrine cells, Schwann cells and natural killer (NK) cells, and can be used to identify neuroendocrine neoplasms or specific NK and T-cell lymphomas (8). TTF-1 is a nuclear stain predominantly seen in lung and thyroid neoplasms, but can display cytoplasmic staining in some hepatocellular carcinomas (8). ER and progesterone receptor (PR) are often used to evaluate breast, ovarian and endometrial neoplasms, but they can be positive in non-gynecologic tumors such as gastrointestinal tumors (8). GATA-3 is expressed in breast epithelium and urothelium, but can be positive in a small percentage of other tumors such as hepatic, pancreatic and gastric carcinoma (8).
Morphologically and immunophenotypically, the tumor appeared to be extrahepatic in origin, most likely from the pancreas; however, CA19-9 was not elevated, and no pancreatic lesions were detected on CT scan of the abdomen.
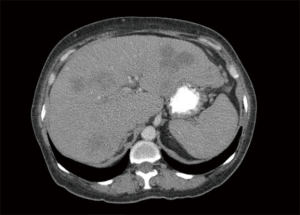
The decision was made initially to treat the tumor as pancreaticobiliary cancer. She was started on gemcitabine and paclitaxel with treatment cycles every 2 weeks and scheduled for serial CT scans to follow the response to therapy. After 8 months, the treatment was transitioned to every 3 weeks for better tolerance. At the time of the preparation of this case report, the patient had been treated for seventeen months. The liver nodules on repeat CT scans (Figure 4) have decreased dramatically in size and number, and her abdominal pain had resolved.
Discussion
ACCs are rare tumors that are usually found in exocrine glands such as pancreas and have the potential to metastasize to other organs (5). ACC involving the liver is most commonly metastatic, typically originating from the pancreas (3,5). In a study of 28 cases of ACC of the pancreas, 13 cases had metastases to the liver and 5 had metastases to lymph nodes (5). However, there have been a limited number of cases reporting ACCs with the liver as a primary source of malignancy.
In a case reported by Hervieu et al., a 35-year-old male patient presented with similar complaints as our patient (9). In their case, the patient’s CT of the abdomen showed a large hepatic mass. No laboratory abnormalities or imaging findings pointed towards any primary source of the cancer (9). Similar to Hervieu et al., we also came to the conclusion of primary ACC of the liver based on histological, immunohistochemical and imaging reports (9). In this last case report, the patient’s primary hepatic ACC was treated with surgical excision. Following surgery, the patient’s previously elevated alpha-fetoprotein (AFP) returned to normal values and for approximately 6 years no evidence of recurrence was noted by the authors. Another case report by Wildgruber et al. focused on the imaging findings of primary hepatic ACC (6). They described multifocal lesions within the liver similar to the findings of our patient’s CT scan. In this particular case the patient underwent palliative chemotherapy because of extensive metastases to the lymph nodes and lung, and went on to live for 18 months (6). The authors also suggested the possibility of ectopic pancreatic acini-like tissue in the liver as a potential hypothesis for primary ACC of the liver (6). In a paper published by Terada et al., 41 out of 1,000 autopsied livers had traces of heterotopic pancreatic tissue (10). Another hypothesis put forth in case reports by Jordan et al., Hervieu et al., and others is that liver and pancreatic cells are ontogenetically derived from a common progenitor cell, leading to the possibility of liver cells differentiating into pancreatic acinar cells (3,9,11,12).
As ACC is a relatively rare entity, there is a lack of treatment protocol and available data, thus much of our understanding comes from case reports. According to a case reported by Aqel et al., the treatment choice for pancreatic ACC depends on the stage of the disease (13). Aqel et al. and Holen et al. recommend surgical resection as first-line treatment for localized and non-metastatic disease (4,13). Adjuvant chemotherapy and radiotherapy have also been mentioned in a few studies as potential treatment regimens for pancreatic ACC, however their efficacy is unclear (14). A retrospective study by Holen et al. concluded that patients with pancreatic ACC who underwent surgical resection had the best outcomes followed by the patients treated with radiotherapy (4). Unfortunately, those treated with chemotherapy had poor results with only 2 out of 18 patients showing partial response to two different chemotherapy regimens (4). One of the regimens contained cisplatin, cytarabine, and caffeine (4). Another consisted of irinotecan, 5-fluorouracil (5-FU) and leucovorin (4). In a review by Chaudhary and Al-Hader et al., the authors suggest that due to the lack of data for pancreatic ACC, many of the therapeutic regimens used for pancreatic ACC are similar to the regimens for colorectal and pancreatic ductal adenocarcinomas because of close genetic alterations noted in these cancers (1,14). A retrospective study by Lowrey et al. showed that about 50–60% of the patients diagnosed with pancreatic ACC with metastases showed response to various combinations of chemotherapy agents such as gemcitabine, docetaxel, cisplatin, irinotecan, and erlotinib (15). In a case report by Antoine et al., a patient with pancreatic ACC with metastasis to the liver was treated with different combinations of chemotherapy agents including cycles of gemcitabine, irinotecan, oxaliplatin, docetaxel, capecitabine, octreotide, leucovorin, 5-FU, cisplatin, protein kinase C inhibitor, tyrosine kinase inhibitor, and a novel taxane. The patient subsequently lived 37 months after the initial diagnosis (16).
Studies reporting the use of transarterial embolization or chemoembolization in pancreatic and hepatic ACC are limited. In a case reported by Riediger et al., treatment of liver metastases from ACC with lipase hypersecretion syndrome was successfully treated with transarterial chemoembolization (TACE) after the patient had failed systemic chemotherapy (17). In the case reported by Jordan et al., the patient’s primary hepatic ACC was treated with a combination of surgical resection, hepatic arterial embolization, and chemotherapy with FOLFOX (combination of folinic acid, 5-FU and oxaliplatin) (3). Twenty months after the initial diagnosis, decreasing necrotic masses were noted on the imaging (3). More research and cases will need to be reported in order to assess the efficacy and long term benefits of different treatment modalities for ACC with metastases to the liver and primary hepatic ACC.
With these previous cases available and our clinical judgement, we believe the patient in our case has primary ACC of the liver. The arguments and findings that support our assessment include the histopathology showing highly differentiated acinar cells, findings on the immunohistochemical stain, radiographic images of the liver masses and the lack of lesions observed in other organs. At the time of submission of this article, it has been seventeen months since systemic chemotherapy consisting of gemcitabine and paclitaxel was initiated, and the patient’s follow-up imaging has demonstrated a positive response with the liver masses decreasing in size.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Chaudhary P. Acinar Cell Carcinoma of the Pancreas: A Literature Review and Update. Indian J Surg 2015;77:226-31. [Crossref] [PubMed]
- Al-Zaher N, Obeid A, Al-Salam S, et al. Acinic cell carcinoma of the salivary glands: a literature review. Hematol Oncol Stem Cell Ther 2009;2:259-64. [Crossref] [PubMed]
- Jordan EJ, Basturk O, Shia J, et al. Case report: primary acinar cell carcinoma of the liver treated with multimodality therapy. J Gastrointest Oncol 2017;8:E65-72. [Crossref] [PubMed]
- Holen KD, Klimstra DS, Hummer A, et al. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol 2002;20:4673-8. [Crossref] [PubMed]
- Klimstra DS, Heffess CS, Oertel JE, et al. Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am J Surg Pathol 1992;16:815-37. [Crossref] [PubMed]
- Wildgruber M, Rummeny EJ, Gaa J. Primary acinar cell carcinoma of the liver. Rofo 2013;185:572-3. [Crossref] [PubMed]
- Kandalaft PL, Gown AM. Practical Applications in Immunohistochemistry: Carcinomas of Unknown Primary Site. Arch Pathol Lab Med 2016;140:508-23. [Crossref] [PubMed]
- Bishop JA, Duffield A, Molavi D, et al. Immunostains: Antibody Index. In: Rekhtman N, Bishop JA. Quick Reference Handbook for Surgical Pathologists. Berlin, Heidelberg: Springer Berlin Heidelberg, 2011:55-68.
- Hervieu V, Lombard-Bohas C, Dumortier J, et al. Primary acinar cell carcinoma of the liver. Virchows Arch 2008;452:337-41. [Crossref] [PubMed]
- Terada T, Nakanuma Y, Kakita A. Pathologic observations of intrahepatic peribiliary glands in 1000 consecutive autopsy livers. Heterotopic pancreas in the liver. Gastroenterology 1990;98:1333-7. [Crossref] [PubMed]
- Deutsch G, Jung J, Zheng M, et al. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development 2001;128:871-81. [PubMed]
- Meivar-Levy I, Ferber S. New organs from our own tissues: liver-to-pancreas transdifferentiation. Trends Endocrinol Metab 2003;14:460-6. [Crossref] [PubMed]
- Aqel B, Scolapio J, Nguyen J, et al. Recurrent pancreatitis due to a cystic pancreatic tumor: a rare presentation of acinar cell carcinoma. JOP 2004;5:151-4. [PubMed]
- Al-Hader A, Al-Rohil RN, Han H, et al. Pancreatic acinar cell carcinoma: A review on molecular profiling of patient tumors. World J Gastroenterol 2017;23:7945-51. [Crossref] [PubMed]
- Lowery MA, Klimstra DS, Shia J, et al. Acinar cell carcinoma of the pancreas: new genetic and treatment insights into a rare malignancy. Oncologist 2011;16:1714-20. [Crossref] [PubMed]
- Antoine M, Khitrik-Palchuk M, Saif MW. Long-term survival in a patient with acinar cell carcinoma of pancreas. A case report and review of literature. JOP 2007;8:783-9. [PubMed]
- Riediger C, Mayr M, Berger H, et al. Transarterial chemoembolization of liver metastases as symptomatic therapy of lipase hypersecretion syndrome. J Clin Oncol 2012;30:e209-12. [Crossref] [PubMed]


