Proton beam therapy versus stereotactic body radiotherapy for hepatocellular carcinoma: practice patterns, outcomes, and the effect of biologically effective dose escalation
Introduction
Now the second leading cause of cancer-related death worldwide, hepatocellular carcinoma (HCC) is also increasing in incidence and mortality in the United States (1,2). Liver transplant remains the only curative option, but the majority of patients fail to meet the surgical or medical criteria for transplant that carries a considerable mortality rate if patients are not properly selected (3). Transarterial chemoembolization (TACE) has been the recommended modality for local control as a bridge to transplant, or in lieu of it for non-surgical patients (4,5). However, growing evidence suggests that external beam radiotherapy, through the advent of stereotactic body radiation therapy (SBRT), challenges this conventional paradigm and provides patients with additional options to achieve durable local control and improved survival (6-9).
Historically, the use of external radiation has been limited in HCC because of the low tolerance to large volumes of irradiated liver parenchyma until researchers at the University of Michigan established that high dose to limited volumes of liver can be delivered with limited risk of radiation-induced liver disease (RILD) (10). Since SBRT optimizes conformal dose distribution, it has emerged as a promising option. Further developing the principle of high dose to limited treatment volumes, proton beam therapy (PBT) contains unique physical properties such as a finite range of dose deposition and sharp lateral penumbra that produces dosimetric advantages relative to photon SBRT (11). This is also valuable for tumors abutting the bowel or hilum, where such structures often serve as dose-limiting organs with SBRT. Early results from clinical trials suggest potentially clinically meaningful outcome benefits with PBT over TACE, although the data still require maturation (12).
Despite the emerging evidence, neither SBRT nor PBT have been widely used in the United States for HCC relative to other parts of the developed world (13,14). As a result, randomized or even larger-volume retrospective data comparing SBRT and PBT for HCC remain altogether non-existent in the American literature. Herein, we aimed to identify the practice patterns and outcomes of nonsurgical HCC cases treated definitively with either SBRT or PBT using the National Cancer Database (NCDB).
Methods
Patient selection
This study was exempt from institutional review board supervision due to the utilization of de-identified data provided by the NCDB, a tumor registry jointly managed by the American Cancer Society and American College of Surgeons. The database captures approximately 70% of cancer cases in the United States from over 1,500 hospitals accredited by the Commission on Cancer (15). We queried the database to identify nonsurgical T1–T2N0M0 HCC patients treated with either SBRT or PBT between the years 2004–2015. A complete CONsolidated Standards Of Reporting Trials (CONSORT) diagram depicting the cohort selection process is outlined in Figure 1. Patients were excluded if they underwent surgical resection or transplant, received a palliative (<30 Gy in 5 fractions) or unknown dose of radiation, or had unknown follow-up. There was no minimum follow-up time required for inclusion to account for immortal time bias so long as treatment was completed, because acute RILD resulting in fulminant hepatic failure has been reported in the literature (16).

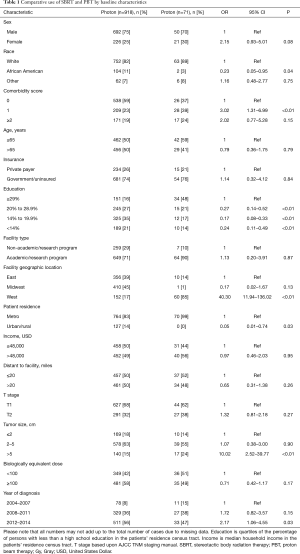
Race was defined as either white, African American, or other/unknown. Comorbidities were quantified via Charlson/Deyo comorbidity index, and stage was defined by American Joint Cancer Committee 7th edition clinical staging. Income data in the patients’ residence census tract were provided as quartiles and reported here as above or below the median. Population classification was based on typology published by the USDA Economic Research Service, facility type was assigned according to Commission on Cancer accreditation category, and insurance status was reported on the admission page.
Statistics
Statistical analysis was performed via Medcalc version 18, with the methodology reported elsewhere (17-19). Summary statistics were reported for discrete variables, and binomial multivariable logistic regression was used to compare socioeconomic, clinical, and treatment characteristics between the SBRT and PBT groups. Overall survival (OS) was calculated from the date of diagnosis to the date of death or censored at last contact using Kaplan-Meier methodology. Multivariable survival analysis was performed for all characteristics listed on Table 1 by first performing independent univariate survival analyses, and statistically significant factors were then entered in a hierarchical fashion using “enter” selection of the covariates’ likelihood ratios. Adjusted hazard ratios (HR) and 95% confidence interval (CI) are reported, with α=0.05 used to indicate statistical significance.
Full table
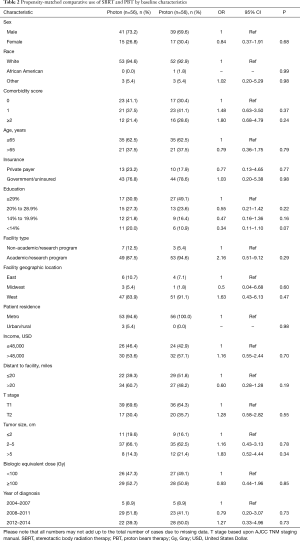
Propensity score analysis was used to account for indication bias caused by lack of randomization (20-22). Propensity scores were calculated by multivariable logistic regression to provide a score reflecting the conditional probability of undergoing SBRT or PBT. The propensity model included observable variables significantly associated with SBRT/PBT selection on multivariable logistic regression, including race, comorbidity score, education level, facility type, region, population density, tumor size, and year of diagnosis. Subsequently, we constructed a pseudo population using the case control function with exact matches based on calculated propensity score, yielding a matched population of 56 patients in each treatment group. To strengthen the assumption of balance between groups, a bivariate regression analysis was performed for all variables included in the propensity-matched analysis, confirming no differences between SBRT and PBT groups (Table 2).
Full table
Results
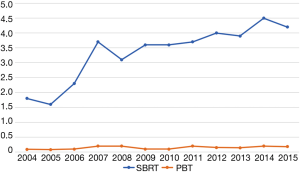
Ultimately, 989 patients were eligible for final analysis, including 918 patients treated with SBRT and 71 with PBT. A comprehensive report of demographic, tumor, and treatment-related characteristics is given in Table 1. The vast majority of patients were white males with a median age of 65 years. Most lesions were T1 (67%) with a median diameter of 3.2 cm [interquartile range (IQR), 2.4–4.7 cm]. Although the laboratory values were reported in only approximately half of all cases (and, therefore, not included in the statistical analysis), the median INR for both SBRT and PBT patients was 1.3 (none exceeding 2.2) and the median total bilirubin for each group was 2.0 mg/dL (none exceeding 3.5 mg/dL). As a percentage of nonsurgical early stage HCC diagnoses per year, SBRT was utilized in 1.8% of cases in 2004, increasing to 4.2% in 2015 (OR =2.66, P=0.02). PBT however, was utilized between 0.1% and 0.2% of cases each year between 2004–2015 (Figure 2).
There was no difference in dose delivered between SBRT and PBT (OR =0.70, P=0.17), with the median biologically effective dose10 (BED) being 100 Gy10 (IQR 79.2–124.8 Gy10) for SBRT and 98 Gy for PBT (IQR 98–113 Gy10). Of note, the median number of fractions for SBRT was 5, compared to 15 for PBT. A BED ±100 Gy10 was elected as the cutoff value for comparison with multivariable analysis because it was both the median value for the entire cohort and the a priori value determined by receiver operating characteristic analysis.
Patients were more likely to receive PBT if they were white, had higher comorbidity scores, higher education, treated in Western regions, located in a metropolitan community, had tumors over 5 cm, or treated more recently (all P<0.05). Following propensity-matched case control, there were no differences between any of the observable characteristics, demonstrated on Table 2.
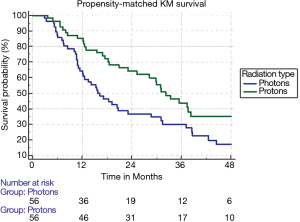
The median follow-up for the entire cohort using the reverse Kaplan-Meier method was 44.8 months (IQR 29.9–65.5 months), with an overall median survival of 25.5 months (95% CI: 22.8–27.8). Patients treated with SBRT had a median survival of 25.2 months (95% CI: 22.4–27.5) compared to 31.0 months (95% CI: 20.6–37.4). With propensity matching, the Kaplan-Meier median survival for SBRT and PBT was 15.7 and 32.2 months, respectively (HR =1.77, 95% CI: 1.14–2.80). The 1- and 3-year survival for SBRT were 64.3% and 30% compared to 76.5% and 36.7% for PBT (P=0.01) (Figure 3). With non-propensity matched multivariable cox regression analysis, the independent predictors for longer survival included “other” race, tumors smaller than or equal to 2 cm, and BED ≥100 Gy10. PBT trended towards longer survival in the non-propensity matched analysis as well (P=0.07). Multivariable analysis within the propensity matched population demonstrated that only PBT (HR =0.48 95% CI: 0.29–0.78) and BED ≥100 Gy10 (HR =0.61, 95% CI: 0.38–0.98) correlated with longer survival (Table 3). BED was also an independent predictor of longer survival as a continuous variable with and without propensity matching (HR =0.99, P<0.001).
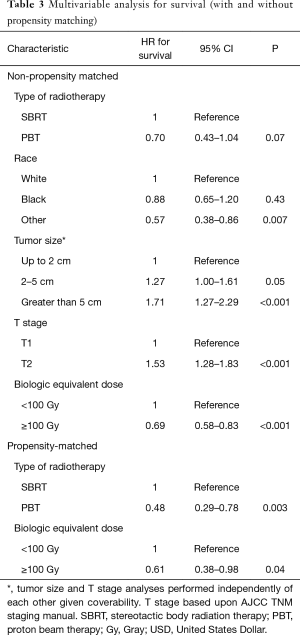
Full table
Discussion
To our knowledge, this is the largest pooled analysis comparing the use of PBT with SBRT in unresectable early stage HCC. Following propensity matching, PBT was associated with increased survival over the more commonly utilized SBRT, despite the former having been delivered to patients with multiple poorer prognostic factors such as higher comorbidities and larger-volume disease. These novel findings imply that PBT may serve as an indirect means to allow for safer BED escalation, which independently associated with outcomes.
Qi et al. published a systematic review that included 1,627, 1,473, and 2,104 HCC patients treated with charged particles, SBRT, and 3D conformal radiation, respectively (13). The vast majority of these patients came from Asia and Europe, underscoring the underutilization of radiotherapy for HCC in the United States. Indeed, approximately 6,000 out of 171,000 patients (3.5%) in the entire HCC dataset had some form of external beam radiation, with a marginal increase over the 12-year period assessed. This is despite the fact that prospective evidence demonstrates equivalent if not superior outcomes with ablative radiotherapy in HCC compared to TACE, albeit not in randomized phase III trials (6,23,24). Also noteworthy, nearly one-third of the patients in the meta-analysis by Qi et al. were treated with particle beam therapy (13), compared to 7% in our final cohort. Certainly, the availability and accessibility of proton therapy currently limit its utilization, and socioeconomic factors typically play a large role in determining the latter (25,26). Importantly, African-American, lower educated, and urban/rural patients were less likely to receive PBT for HCC. One might presume that given this demographic selection bias, PBT patients were also healthier with lower disease burden, but in fact the opposite was true—patients with higher comorbidity scores and tumors larger than 5 cm were considerably more likely to be treated with PBT compared to SBRT. This also likely explains, in part, why for the propensity-matched analysis PBT significantly correlated with longer survival.
Given the inherent selection bias and inability to control for unobservable variables with propensity matching, the difference in survival should be interpreted with caution. Nevertheless, it is possible that dosimetric advantages with PBT, specifically as it pertains to the liver, may lead to improved outcomes. Phase III trials have constrained the liver V15 (in 5 fractions) to be less than 700 cc and the mean dose under 13–17 Gy (based on fractionation) (27). Although SBRT can often achieve these constraints, this becomes more challenging for larger tumors surrounded by high volumes of normal liver (i.e., located centrally or at the dome) (11,28). One dosimetric analysis demonstrated that for tumors greater than 3 cm and/or located at the dome of the liver, the normal liver volume irradiated and mean liver dose were reduced by an average of 176 cc and 4 Gy, respectively, with PBT compared to SBRT (29). This may further help to explain why there was an equivalent median BED delivered for both PBT and SBRT patients, despite larger tumors and more comorbid patients included in the PBT cohort, as some patients across both groups may have been treated with risk-adaptive dosing based on the volume of liver irradiated. Unlike some other disease sites where the volume of low dose irradiation distribution is clinically irrelevant, most HCC patients have liver dysfunction at baseline, so any excess amount of treated tissue may be consequential.
The ability to safely escalate dose/BED in HCC, as SBRT has done relative to conventional radiation, may be the avenue to better local control and consequently improved survival. For the reasons mentioned above, PBT can potentially further increase this therapeutic ratio. Arscott et al. demonstrated in a dosimetric analysis that liver tumors between 1–10 cm received on average a 6.3-Gy higher integral dose with a simultaneous 4.9 Gy mean liver dose reduction with stereotactic PBT compared to photon-based SBRT (28). In another retrospective study of 79 patients with intrahepatic cholangiocarcinoma, a BED of 80.5 Gy was associated with a 3-year survival of 73% compared to 44% under 80.5 Gy10 (29). In this current study, a significant independent predictor of survival was a BED >100 Gy10 (HR =0.69, P<0.001), which was delivered at approximately the same rate between SBRT and PBT, despite the latter treating a larger volume on average. Although non-OS endpoints are not captured by the NCDB, the aforementioned non-NCDB study (30). did associate BED with both local control and OS, thus implying that the OS endpoint in this investigation is a valid one. Taken together, when interpreting these data conservatively given retrospective biases in PBT versus photon treatment, we posit that even if there is no direct effect of PBT on survival, it may exert a secondary effect by allowing for safer BED escalation.
Relative to historic controls, PBT has demonstrated a low toxicity profile with no instances of RILD in phase I trials (31,32). This has also held true for several SBRT studies, although the incidence of RILD increases for Child Pugh B patients (33,34). Although toxicity data is also unreported, one must consider the possibility that one of the reasons for worse survival despite seemingly more favorable tumors in the SBRT group was due to a higher rate of radiation induced liver disease. While no randomized study comparing SBRT with PBT in HCC exists to date, observational studies on average demonstrate a comparatively lower toxicity profile with PBT, albeit without a notable difference in control or survival (13). However, a phase III trial by Bush et al. demonstrated a trend to superior loco-regional control and lower toxicity with PBT compared to TACE for HCC (12). Unfortunately, patterns of failure are not reported in the NCDB, and while there was no difference in survival for the cohort as a whole, a difference in control and toxicity profile may have contributed to the survival difference noted on propensity matched analysis.
Although well powered for a restricted patient population, this study is subject to the selection bias present in all NCDB studies. However, several statistical measures were performed to mitigate the biases caused by lack of randomization. Despite this, it is not possible to account for unobservable variable such as etiology of cirrhosis, patterns of failure, salvage therapies, alpha-fetoprotein levels, tumor location, and Child Pugh/MELD score. Of particular interest in this patient population is baseline liver function (such as measured by Child Pugh score) but this is not reported by the NCDB. This may certainly impact cause of death, which is also not captured by the NCDB. To attempt to control for this, we excluded palliative and locally advanced cases, and we analyzed baseline laboratory values, with no differences in total bilirubin or INR seen.
Conclusions
Despite mounting evidence supporting the expanded role of ablative external beam radiotherapy in HCC, our NCDB analysis demonstrates that utilization, while modestly growing, is still very low. Even less utilized, perhaps due in part to lack of availability and accessibility rather than indication, PBT appears to be another promising modality against HCC. Because higher BED was associated with improved survival both in this study and in prior analyses, and since PBT can allow for safer BED escalation, PBT may be a means to improve clinical outcomes for HCC. Although causation between PBT and survival as observed herein cannot be implied, the effective delivery of ablative doses to larger tumors is consistent with the evolving literature and, therefore, randomized investigation of both modalities, such as with NRG-GI003/NCT03186898, is warranted.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was exempt from institutional review board supervision due to the utilization of de-identified data provided by the NCDB.
References
- Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog 2017;16:1. [Crossref] [PubMed]
- Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am 2015;24:1-17. [Crossref] [PubMed]
- Penn I. Hepatic transplantation for primary and metastatic cancers of the liver. Surgery 1991;110:726-34; discussion 734-5. [PubMed]
- Lesurtel M, Müllhaupt B, Pestalozzi BC, et al. Transarterial Chemoembolization as a Bridge to Liver Transplantation for Hepatocellular Carcinoma: An Evidence-Based Analysis. Am J Transplant 2006;6:2644-50. [Crossref] [PubMed]
- Meyer T, Fox R, Ma YT, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol 2017;2:565-75. [Crossref] [PubMed]
- Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol 2013;31:1631-9. [Crossref] [PubMed]
- Klein J, Dawson LA. Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. Int J Radiat Oncol Biol Phys 2013;87:22-32. [Crossref] [PubMed]
- Sapisochin G, Barry A, Doherty M, et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol 2017;67:92-9. [Crossref] [PubMed]
- Hasan S, Thai N, Uemura T, et al. Hepatocellular carcinoma with child Pugh-A Cirrhosis treated with stereotactic body radiotherapy. World J Gastrointest Surg 2017;9:256-63. [Crossref] [PubMed]
- Ben-Josef E, Normolle D, Ensminger WD, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol 2005;23:8739-47. [Crossref] [PubMed]
- Wang X, Krishnan S, Zhang X, et al. Proton radiotherapy for liver tumors: dosimetric advantages over photon plans. Med Dosim 2008;33:259-67. [Crossref] [PubMed]
- Bush DA, Smith JC, Slater JD, et al. Randomized Clinical Trial Comparing Proton Beam Radiation Therapy with Transarterial Chemoembolization for Hepatocellular Carcinoma: Results of an Interim Analysis. Int J Radiat Oncol Biol Phys 2016;95:477-82. [Crossref] [PubMed]
- Qi WX, Fu S, Zhang Q, et al. Charged particle therapy versus photon therapy for patients with hepatocellular carcinoma: A systematic review and meta-analysis. Radiother Oncol 2015;114:289-95. [Crossref] [PubMed]
- Verma V, Lin SH, Simone CB, et al. Clinical outcomes and toxicities of proton radiotherapy for gastrointestinal neoplasms: a systematic review. J Gastrointest Oncol 2016;7:644-64. [Crossref] [PubMed]
- Winchester DP, Stewart AK, Bura C, et al. The National Cancer Data Base: A clinical surveillance and quality improvement tool. J Surg Oncol 2004;85:1-3. [Crossref] [PubMed]
- Kim J, Jung Y. Radiation-induced liver disease: current understanding and future perspectives. Exp Mol Med 2017;49:e359. [Crossref] [PubMed]
- Hasan S, Renz P, Wegner RE, et al. Microsatellite Instability (MSI) as an Independent Predictor of Pathologic Complete Response (PCR) in Locally Advanced Rectal Cancer. Ann Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Hasan S, Renz P, Turrisi A, et al. Dose escalation and associated predictors of survival with consolidative thoracic radiotherapy in extensive stage small cell lung cancer (SCLC): A National Cancer Database (NCDB) propensity-matched analysis. Lung Cancer 2018;124:283-90. [Crossref] [PubMed]
- Verma V, Ahern CA, Berlind CG, et al. Treatment of malignant pleural mesothelioma with chemotherapy preceding versus after surgical resection. J Thorac Cardiovasc Surg 2019;157:758-66.e1. [Crossref] [PubMed]
- D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265-81. [Crossref] [PubMed]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaum, 1988.
- Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46:399-424. [Crossref] [PubMed]
- Andolino DL, Johnson CS, Maluccio M, et al. Stereotactic Body Radiotherapy for Primary Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys 2011;81:e447-53. [Crossref] [PubMed]
- Hong TS, Wo JY, Yeap BY, et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J Clin Oncol 2016;34:460-8. [Crossref] [PubMed]
- Shen CJ, Hu C, Ladra MM, et al. Socioeconomic Factors Affect the Selection of Proton Radiation Therapy for Children. Cancer 2017;123:4048-56. [Crossref] [PubMed]
- Mahal BA, Chen YW, Efstathiou JA, et al. National trends and determinants of proton therapy use for prostate cancer: A National Cancer Data Base study. Cancer 2016;122:1505-12. [Crossref] [PubMed]
- Dawson LA, Hospital PM, Zhu A, et al. Radiation therapy oncology group RTOG 1112 randomized phase iii study of sorafenib versus stereotactic body radiation therapy followed by sorafenib in hepatocellular carcinoma study team. Principal Investigator/Radiation Oncology. Available online: https://www.ctsu.org
- Arscott WT, Thompson RF, et al. Stereotactic body proton therapy for liver tumors: Dosimetric advantages and their radiobiological and clinical implications. Phys Imaging Radiat Oncol 2018;8:17-22. [Crossref]
- Gandhi SJ, Liang X, Ding X, et al. Clinical decision tool for optimal delivery of liver stereotactic body radiation therapy: Photons versus protons. Pract Radiat Oncol 2015;5:209-18. [Crossref] [PubMed]
- Tao R, Krishnan S, Bhosale PR, et al. Ablative Radiotherapy Doses Lead to a Substantial Prolongation of Survival in Patients With Inoperable Intrahepatic Cholangiocarcinoma: A Retrospective Dose Response Analysis. J Clin Oncol 2016;34:219-26. [Crossref] [PubMed]
- Bush DA, Kayali Z, Grove R, et al. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer 2011;117:3053-9. [Crossref] [PubMed]
- Allegra CJ, Yothers G, O’Connell MJ, et al. Neoadjuvant 5-FU or capecitabine plus radiation with or without oxaliplatin in rectal cancer patients: A phase III randomized clinical trial. J Natl Cancer Inst 2015. [Crossref] [PubMed]
- Hasan S, Abel S, Jan I, et al. The albumin-bilirubin (ALBI) model in hepatocellular carcinoma (HCC) may better predict hepatic failure in patients with traditionally low-risk cirrhosis following definitive stereotactic body radiotherapy (SBRT). J Radiat Oncol 2018;7:247-53. [Crossref]
- Cárdenes HR, Price TR, Perkins SM, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol 2010;12:218-25. [Crossref] [PubMed]