Outcomes of rectal cancer with liver oligometastases
Introduction
Colorectal cancer is amongst the top three most commonly diagnosed cancers in men and women alike (1). In the United States, nearly 143,000 new cases of colorectal cancer are still diagnosed yearly, roughly 40,000 of which are rectal cancers (2). Rectal and colon cancer combined account for roughly 51,000 deaths a year.
In 1998, a trial was conducted in Sweden in which 1,168 rectal cancer patients were assigned to two treatment groups: surgery alone, or preoperative radiation therapy followed by surgery (3). In this study patients were treated with a short course of radiation therapy, 25 Gy in 5 days. The overall survival (OS) rate in the irradiated group was 38% vs. 30% in the nonirradiated group. The cancer-specific survival rate in the irradiated group was 72% vs. 62% in the nonirradiated group. The greatest difference was seen in the local recurrence rate, which was 9% and 26%, in the irradiated and non-irradiated group respectively. This study provided substantial evidence for the use of radiation therapy in the treatment of rectal cancer.
A similar trial was also conducted in Holland (4), which looked at 1,861 patients assigned to either preoperative radiotherapy (25 Gy in 5 days) followed by total mesorectal excision (TME) or to TME alone. Although OS rates were nearly identical, the rate of local recurrence at 2 years was 2.4% in the radiotherapy and TME group and 8.2% in the surgery alone group, attesting to the importance of radiation therapy in local control (LC) of disease.
Sauer et al. published their landmark trial in 2004 (5). They looked lesions staged at T3-T4, and divided them into two treatment groups, pre and post-surgical radiation therapy with the goal of seeing which group had better survival outcomes. The preoperative treatment consisted of 5,040 cGy delivered in fractions of 180 cGy per day, 5 days per week with surgical resection occurring 6 weeks after completion of treatment. The post-surgical group had a similar treatment approach, with the addition of a 540 cGy boost, and the radiation therapy being started one month after surgery. Although OS was nearly identical, the 5-year incidence of local relapse was 6% for patients assigned to preoperative radiation therapy and 13% in the postoperative radiation therapy group. Thus, pre-operative treatment did not increase survival but led to a marked improvement in LC.
However, the studies above mostly addressed patients with locally advanced disease, not those with metastatic disease. The literature remains scarce as far as these patients’ outcomes go, and how should they be treated. The current literature states that perhaps patient with liver oligometastases should be treated with curative intent (6), as it has been shown that patients with a single liver lesion, after hepatic resection of the lesion, have a 72% 5-year OS rate as well as a 5% local recurrence rate in the liver (7).
In our study we report of a single institution experience of oligometastatic rectal cancer patients after treatment of the primary tumor and pelvic lymph nodes with extended course chemoradiation therapy.
Methods
Before the initiation of therapy, all patients underwent a complete history and physical examination along with necessary imaging such as computed tomography and magnetic resonance imaging. More recently, positron-emission tomography was frequently used in the staging and evaluation of these patients.
Diagnosing and staging followed AJCC guidelines with biopsies and appropriate imaging performed. These patients were all treated with curative intent with radiation therapy. Outcomes data was obtained through extensive review of medical records as well as imaging. Survival outcomes were obtained based on medical records and verified with social security death index.
The start point for patient follow up was when patients were first diagnosed by way of imaging followed by pathology. Distant control (DC) in our study was defined as absence of progression, since the patients did not undergo specific treatment for their liver metastases. Failure dates were determined by either presence of distant metastases (DM) or local recurrence on PET, CT or MRI scans. Statistical analysis was performed using SPSS.
Results
Patients
Between 2004 and 2013, 26 rectal cancer patients presented to New York University’s Perlmutter Cancer Center with metastatic disease. Amongst these there were 17 men and 9 women. The mean age at the time of diagnosis was 59.8 years, with a range from 36 to 87 years of age. Eleven patients had metastases in other sites in addition to liver, and one patient in our cohort had lung metastasis with no liver metastasis. All but two patients received chemo and one patient’s chemo status was unknown due to limited records. Eleven patients received intensity modulated radiation therapy (IMRT) and the rest received the conventional radiation therapy with 3D planning (3D-CRT). The most common dose was 4,500 cGy with a 540 cGy boost, with 16 patients receiving it (Table 1).
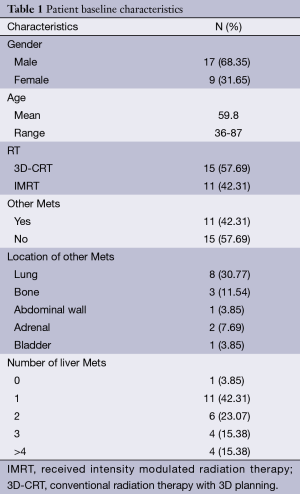
Full table
Survival
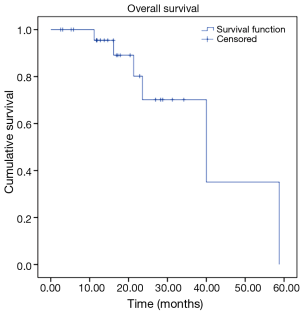
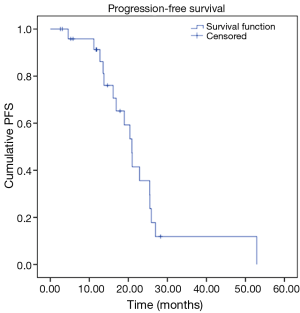
Our analysis revealed the following results in our cohort. We observed OS rates of 95%, and 70% at 12 and 24 months respectively, with a mean survival time of 40.5 months (Figure 1). Our cohort also revealed progression free survival (PFS) rates of 91% and 36% at 12 and 24 months respectively, with a mean PFS time of 23.1 months (Figure 2).
Control
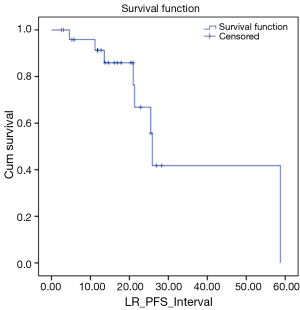
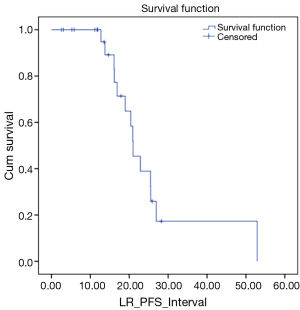
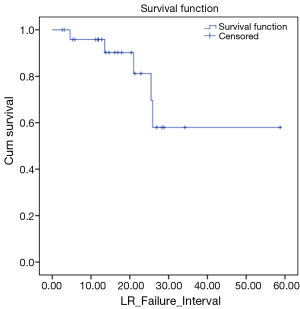
We also looked at how these patients performed in terms of local and DC. LC rates were 91% and 66% at 12 and 24 months respectively (Figure 3). DC rates were 100% and 39% respectively (Figure 4). Finally, when censoring deaths, progression of liver metastases and distant progression, the Kaplan-Meier curve revealed five events of local failure (Figure 5).
Discussion
Outcomes of rectal cancer treated with radiation therapy
In addition to the studies referenced in our introduction in 2001, the Lancet published a meta-analysis looking at data from 22 randomized comparisons with a total of 8,507 patients, to help elucidate the role of radiation therapy in the treatment of locally advanced rectal cancers (8). In this meta-analysis, OS was only marginally better in patients who received radiotherapy than those who did not, with 45% vs. 42.1% alive at 5 years, and 26.9% vs. 25.3% alive at 10 years. However, a much more marked difference was noted when we looked at local recurrences. The patients that received radiation therapy demonstrated much better LC compared with those who did not, with 5-year local recurrence rates of 12.5% vs. 22.2% for those who did not receive RT, and 10-year recurrence rates of 55.1% vs. 60.8% for any recurrence. Also worth mentioning was how studies of preoperative radiotherapy that used low (<20 Gy) biologically effective doses had no significant benefit. Studies in which high (>30 Gy) biologically effective doses were used showed a halving of the risk of local recurrence.
In 2003, Gunderson et al. (9) pooled data from 3,791 patients with rectal cancer, and attempted to look at the effect of treatment method as well as T and N stages in survival and relapse rates. This study revealed a progressive decrease in OS with an increasing T stage, with T1-2 demonstrating a 5-year OS rate of nearly 75%, whereas T4 demonstrated a 5-year OS rate of 47%. The addition of N substages showed a decrease in OS with T1-3 stages, whereas in T4 it remained unchanged. Similar trends were observed for relapse and DM. Radiation therapy or surgery alone were associated with significantly worse outcomes as opposed to other treatment approaches, however a trimodality approach (surgery, chemotherapy and radiation therapy) may not be necessary for lower risk lesions, and should be reserved for higher risk ones.
Outcomes of patients with oligometastatic liver disease
A literature research revealed other studies looking at outcomes of patients with liver oligometastatic disease. In 2012 Dellas et al. (10) conducted a phase I trial which looked at nine patients with liver oligometastases from colorectal cancer who underwent radiation therapy to the liver metastases (3D-CRT) while adjunctively receiving capecitabine and oxaliplatin. A total irradiation dose of 36 or 44 Gy was delivered in 2.0 Gy daily fractions using high-energy photons (6 to 15 MeV) and three-dimensional planning with measurements of the macroscopic tumor, planning target volume (PTV) and organs at risk were mandatory. The clinical target volume (CTV) was the GTV of each lesion. PTV contained all detectable metastases was derived by adding a margin of 10 mm to account the CTV for the setup uncertainties. Of these nine patients, six were treated to 36 Gy and three to 44 Gy. Six of nine patients achieved tumor response. Disease progression after 1 year occurred in three patients. One year after initiation, all patients were still alive. Within the following year, three patients died from tumor progression. Further studies on the role of radiation in treating liver oligometastases are warranted.
In 2004 Adam et al. (11) looked at a cohort of 1,104 patients with unresectable colorectal liver metastasis. Among these, 138 “good responders” (12.5%) underwent secondary hepatic resection after an average of ten courses of chemotherapy. The vast majority of these patients received a regimen consisting of 5-FU and Folfox and underwent major hepatectomy subsequently. After a mean follow-up of 48.7 months 80% of the patients developed tumor recurrence. However, survival was 52%, 33%, and 23% at 3, 5, and 10 years, with a disease-free survival of 30%, 22%, and 17%, respectively. No mention of the patients receiving radiation therapy is made in this paper.
In 2005, Ben-Josef et al. (12) conducted a phase II trial to determine if to determine if high-dose radiation with concurrent hepatic arterial floxuridine would improve survival in patients with unresectable intrahepatic malignancies. A total of 128 patients underwent 3D-CRT as well as hepatic arterial floxuridine. The radiation was administered as follows: The CTV included the GTV plus a 1-cm margin within the liver. The PTV included the CTV plus a 0.5-cm uniform expansion for setup uncertainty, plus an additional 0.3 to 3 cm margin in the craniocaudal dimension to account for breathing motion. The prescribed dose to the isocenter ranged from 40 to 90 Gy with a median, of 60.75 Gy. The results seemed promising with the actuarial 3-year OS of 17% and the median survival was 15.8 months from the start of RT. This treatment approach also showed an improvement from 8-9 months median survival rates for those patients ineligible for resection.
Pre vs. post-operative radiation therapy
In 2012 a follow up study (13) on the Sauer et al. paper (5) compared outcomes of patients treated with pre-operative versus post-operative chemoradiation. Disease free survival at the 11-year mark showed a negligible difference of only 1% in favor of the pre-operative arm. A similar negligible result was found regarding distant metastasis. A more noticeable difference was seen in the cumulative incidence of local recurrence at 5 and 10 years in this intention-to-treat population. The 5- and 10-year rates were 5% and 7.1% in the group assigned to preoperative CRT and 9.7% and 10.1% in the group assigned to postoperative CRT. Studies such as these provide support for the use of pre-operative CRT in the treatment of rectal cancer.
Prognostic factors
Several studies looked at prognostic factors. One such study (14), showed that the only independent prognosis factor for liver metastases was tumor regression grade, whereas tumor regression grade, low rectal location, and previous liver metastasis predicted lung recurrence.
New approaches and shorter courses
Ayez et al. (15) sought to determine the effects of a “liver first” approach when treating liver oligometastases patients. In this approach, the liver metastases were treated before the primary lesion. If the response was deemed adequate, the patient would go on to complete neoadjuvant chemoradiation followed by surgery. Although limited by a small sample size (only 31 patients actually completed the “liver first” approach), the study showed promising results, with 74% of these patients undergoing subsequent curative treatment for their rectal cancer lesions, showing a 5-year OS of nearly 67%.
In early 2013, the Journal of Clinical Oncology published the outcomes of TROG 01.04. (16) which looked at patients with rectal adenocarcinoma T2-T3 treated with either 5 factions of 5 Gy in 1 week followed by early surgery vs. 50.4 Gy in 1.8 Gy fractions over 5.5 weeks with surgery following in 4 to 6 weeks. Three-year local recurrence rates were 7.5% and 4.4% for the short and long treatment regimen groups respectively. OS rates at 5 years were 74% and 70% for the short and long treatment groups respectively. Although, it seems like local recurrence rates seem slightly worse for the shortened treatment group we cannot dismiss it as an effective alternative to the more conventional hyperfractionated treatment.
The outcomes of a short course of radiation therapy followed by chemotherapy and subsequent surgery for stage IV rectal cancer was looked at by van Dijk et al. (17). In this study patients received five fractions of 5 Gy followed bevacizumab, oxaliplatin, and capecitabine for as many as 6 cycles. Surgery was then carried out 6-8 weeks after the last bevacizumab dose. Among these 50 patients, 84% had liver metastases, 10% had lung metastases, and only 6% had both liver and lung metastases. These patients showed an 80% OS rate and 2-year recurrence rates of 64% compared to 70% and 66% for our cohort. It worth pointing out that our cohort on average had more advanced disease.
This shorter treatment approach is currently being investigated by other studies as well (18). One randomised clinical trial going on right now, the RAPIDO trial, proposes approach involves the use of five fractions of 5 Gy preoperatively as opposed to the more standard 25 fractions of 2 Gy each with pre as well as post-operative chemotherapy. In addition this experimental short course arm did not include postoperative chemotherapy. We currently await the results as to determine whether a shorter course can be proven to be as effective in the management of these patients.
Conclusions
This series demonstrated an OS of 70% at 24 months, with a mean survival of 40.5 months. Significantly, LC was only 66% despite the use of extended course chemoradiation and TME. Our cohort has demonstrated slightly worse outcomes then the some of the current literature; however our patients on average had more advanced disease than on other similar studies.
Recent data suggests that a hypofractionated radiation regiment of 25 Gy in 5 Gy fractions allows an equivalent LC compared to extended course chemoradiation with 50.4 Gy in 1.8 Gy fractions. A short course of radiation may be more consistent with the goals of care of the oligometastatic rectal cancer patient who is at high risk of recurrence.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Folkesson J, Birgisson H, Pahlman L, et al. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005;23:5644-50. [PubMed]
- Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46. [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [PubMed]
- Zhang ZG, Song C, Wang H. Treatment efficacy of surgical management for liver metastasis from colorectal cancer--a report of 198 cases. Ai Zheng 2006;25:596-8. [PubMed]
- Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 2006;141:460-6; discussion 466-7. [PubMed]
- Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet 2001;358:1291-304. [PubMed]
- Gunderson LL, Sargent DJ, Tepper JE, et al. Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer: a pooled analysis. J Clin Oncol 2004;22:1785-96. [PubMed]
- Dellas K, Reese T, Richter M, et al. Concurrent chemoradiation of metastases with capecitabine and oxaliplatin and 3D-CRT in patients with oligometastatic colorectal cancer: results of a phase I study. Radiat Oncol 2012;7:83. [PubMed]
- Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg 2004;240:644-57; discussion 657-8. [PubMed]
- Ben-Josef E, Normolle D, Ensminger WD, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol 2005;23:8739-47. [PubMed]
- Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33. [PubMed]
- Arredondo J, Baixauli J, Beorlegui C, et al. Prognosis factors for recurrence in patients with locally advanced rectal cancer preoperatively treated with chemoradiotherapy and adjuvant chemotherapy. Dis Colon Rectum 2013;56:416-21. [PubMed]
- Ayez N, Burger JW, van der Pool AE, et al. Long-term results of the “liver first” approach in patients with locally advanced rectal cancer and synchronous liver metastases. Dis Colon Rectum 2013;56:281-7. [PubMed]
- Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30:3827-33. [PubMed]
- van Dijk TH, Tamas K, Beukema JC, et al. Evaluation of short-course radiotherapy followed by neoadjuvant bevacizumab, capecitabine, and oxaliplatin and subsequent radical surgical treatment in primary stage IV rectal cancer. Ann Oncol 2013;24:1762-9. [PubMed]
- Nilsson PJ, van Etten B, Hospers GA, et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer--the RAPIDO trial. BMC Cancer 2013;13:279. [PubMed]