Thermal tumor ablation therapy for colorectal cancer hepatic metastasis
Introduction
The optimal clinical role of thermal based tumor ablation modalities (TTA), including cryoablation, radiofrequency, and microwave ablation, in the treatment of colorectal hepatic metastases (CRHM) has been a topic of discussion and investigation for the last two decades. The appropriate indications for TTA and how to best integrate TTA with other regional and systemic modalities are issues surrounded by considerable controversy. Succinctly put, the authors propose that the volume of work that has been performed in the field of CRHM would support the following; (I) the gold standard is that CRHM should be resected when appropriate conditions are met; (II) combined resection-ablation treatment is reasonable when tumor can be completely cleared; (III) staged resection in combination with TTA is a reasonable option when preservation of sufficient parenchyma would not be possible with resection alone; and (IV) TTA modalities can be used as components of consolidation therapy for patients ultimately determined to have unresectable disease.
Approximately 50% of patients with node-positive colorectal cancer (CRC) will eventually develop liver metastases during their disease process (1,2). The median survival of patients with CRHM without any treatment is 8 to 10 months, and 5-year survival is less than 5% (3,4). With the use of modern combination systemic therapy regimens, median overall survival has increased to 20-24 months (5-8). For selected patients, who undergo surgical resection, a 5-year survival as high as 58% has been reported (9-11). Thus, surgical resection is the gold standard for treatment for patients with CRHM and the only potentially curative therapy.
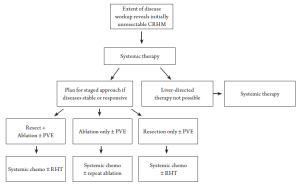
Unfortunately, approximately 80-90% of patients with CRHM are not candidates for surgical resection with intent to cure due to various limitations, including: the presence of extra-hepatic metastases, unfavorable anatomic location of tumor(s), estimated insufficient post-resection functional hepatic reserve (<25-30%), or prohibitive medical co-morbidities. In addition, modern chemotherapy regimens involving oxaliplatin may limit the functional capacity of the liver remnant due to hepatotoxicity (12,13) (Figure 1). Thus, for the majority of patients, only non-surgical treatment options are available and these can be broadly categorized as systemic therapies or regional hepatic therapies (RHT). Systemic therapies include chemotherapy and/or biologic agents alone or in combination with other modalities, which are intended to downstage initially unresectable metastases, reduce or stabilize the disease burden in the liver for previously chemotherapy naïve patients at high risk for progression, or as sole treatment for clearly advanced unresectable disease.
RHT can be further grouped into catheter based treatments such as trans-arterial chemo-embolization (TACE, DEBS, etc), radio-embolization (Ytrrium-90) or immune therapies, such as those offered at our center; regionally infused genetically modified T cells. The other RHT are the local tumor-ablative therapies such as radiofrequency ablation (RFA), microwave ablation (MWA). In recent years, local ablative therapies have become one of the most widely employed modalities to treat initially unresectable CRHM. RFA and MWA are “hot” thermal tumor-ablation treatments that can be used as a component of the initial resection, as components of staged resection, with or without systemic therapy or as standalone treatment strategies for patients with CRHM whom are not initially candidates for resection or are ultimately determined to be unresectable. RFA and MWA are important components of the armamentarium for treating CRHM when surgical resection with negative margins, the gold standard, is not possible due to oncologic, physiologic, or anatomic reasons.
A brief primer on ablation modalities
For the purposes of this discussion, ablation will be limited to “hot” thermal ablation; and thus focused on RFA and MWA. Because MWA is a more recent addition to the surgeon’s armamentarium, the discussion will proceed from the perspective of the RFA literature and except for several caveats, which differentiate RF from MW energy, the assumption is made that the clinical performance of MWA is at least that of RFA. Our discussion will not include “cold” thermal ablation (cryoablation), chemical ablation (percutaneous ethanol injection, acetic acid injection, etc) electrical ablation (irreversible electroporation, IRE), or high intensity focused ultrasound (HIFU) as RFA and MWA are the most commonly utilized technologies at the present time.
Radiofrequency Ablation
RFA induces tumor necrosis by achieving local hyperthermia with temperatures exceeding 58°C. RFA is based on alternating current of radio frequency waves (≈500 KHz) that are transmitted via a probe into tissue to cause ionic agitation, which generates frictional heat that extends into adjacent tissue by conduction. Eventually, hyperthermia leads to cell destruction as a result of coagulative necrosis (14). RFA can be performed under US, CT, or MRI guidance. This can be achieved by percutaneous, laparoscopic, or open surgical approaches, depending on operator preference, tumor anatomy, and extent of disease. However, multiple studies (15-18) have shown that the open surgical approach is superior to percutaneous approach in terms of minimizing local recurrence rates. Better exposure of the liver, ability to visually inspect and palpate surface liver lesions, and ability to use intra-operative ultrasound with its associated high sensitivity to detect additional lesions may explain the superior results of surgical approach (19-21).
Limitations of RFA
Tumor number and tumor size are important determinants of local recurrence rates or treatment failure after RFA. Patients with solitary CRHM have been shown to have better survival and lower recurrence rates compared to those with multiple CRHM (22,23). Similarly, patients with tumors of size less than or equal to 3 cm have better recurrence free survival following ablation (16,24,25). The optimal negative margin size or ablation zone extension beyond the tumor border for RFA of CRHM has not yet been standardized. Currently, ablating to a negative margin of 0.5 - 1 cm has been recommended (15,20). On the other hand, one study (26) has showed that the rate of local tumor progression was independent of the size of the post-ablation margin, and a meta-analysis (21), suggested that 1 cm intentional margin was not a significant factor on multivariate analysis, for local recurrence However, there is no disagreement that complete eradiation of tumor cells in the target lesion(s) is primary goal of any attempt at ablation. Reported rates of local recurrence from RFA for CRHM range widely, from 2% to 40% (10,20,27).
A well recognized cause of RFA treatment failure is the location of CRHM in close proximity to blood vessels. In this scenario, complete tumor ablation has proven to be challenging as the result of “convective heat loss”, or heat sink effect, as it commonly referred (21,28,29), in which thermal energy produced by ablation is shunted away from the tumor by the cooler blood, and higher electrical conductivity of blood (30) that, also, carries heat away from tumor. This specific limitation can be potentially overcome by occluding the hepatic inflow with a Pringle maneuver (28,31,32). However, the Pringle maneuver has to be used with caution when performing RFA, as there is a risk of hepatic vein and portal vein thrombosis (33). RFA also has technical and mechanical limitations (34), including the challenges of targeting isoechoic lesions using ultrasound-guidance. Moreover, CT- or US-guided RFA is time consuming, as complete destruction of a 4 cm lesion can take up to 30 minutes (35). The visualization of liver tumors on standard B-mode sonography may be improved with contrast enhancement using perfluorocarbon microbubbles (36).
Microwave Ablation (MWA)
Like RFA, MWA is also a “hot” thermal ablation modality. MWA uses microwave frequencies >900 MHz (up to 2.4 GHz). The electric charge from MW radiation interacts with water molecules, causing them to oscillate and agitate, producing friction and heat, thus producing cellular death by coagulative necrosis (37,38). MWA has many similarities with RFA in terms of patient selection and technique. Probe placement can be achieved, like RFA, by percutaneous, laparoscopic, and open surgical approaches. Advantages of the surgical approach over percutaneous access are as discussed previously. Due to the relatively recent availability of MWA there is a lack of mature data that can be independently assessed.
Advantages of MWA vs. RFA
MWA has multiple advantages over RFA that include wider ablation diameter, higher ablation rates, avoidance of the heat sink effect (39,40), and shorter duration of ablation. Unlike RFA, MWA does not need a grounding pad, thus eliminating a source of skin burns. MWA can simultaneously utilize multiple probes for ablation, thus ablating larger volumes of tissue in shorter periods of time. As opposed to conductive heating in RFA, MWA involves active heating, which causes cellular destruction throughout the entire microwave field. Unlike RFA, MWA is not affected by charred and desiccated tissue at the tip of probe due to active heating, and thus produces more uniform and reliable tissue ablation zones (40,41). Limitations of MWA include the higher probe costs and diameters, the latter of which may lead to visceral or vascular trauma (endothelial damage, portal vein thrombosis) (42,43).
Summary
This brief synopsis on thermal tumor ablation highlights the characteristics of RFA and MWA, which provides the rationale for inclusion of ablation as an adjunct to resection, with or without systemic therapy or as the primary modality for patients with CRHM whom are not initially candidates for resection or are ultimately determined to be unresectable, as discussed below.
Resection Combined with Ablation
This section heading is deliberately vague. It would be well beyond the scope of this article to include all of the major patient management controversies that emerge when considering the innumerable potential clinical scenarios that may arise when treating patient with CRHM not clearly amenable to surgical resection at the time of presentation. Our discussion will not address the topic of selecting patient for neoadjuvant or perioperative systemic therapy. The other significant issue that will not be covered in detail is the ongoing debate regarding the timing of primary tumor resection in relation to systemic therapy and liver resection or tumor ablation.
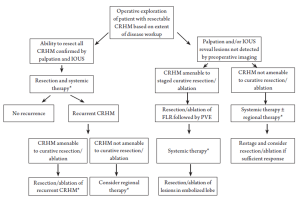
For patients who present with CRHM that cannot be initially managed by resection alone, formulation of an individualized multimodality treatment plan for each patient is imperative (Figure 2). This treatment plan will by necessity vary depending on the biology of disease, anatomic considerations, and overall physiology of the patient. For example, a patient at high risk for post-resection disease progression, as indicated by important surrogates of outcome including the clinical risk score components (44), may be considered for non-ablative regional hepatic therapies (45), such as lobar or whole liver yttrium-90 infusion which may scheduled before or after systemic chemotherapy. Infusional brachytherapy may achieve sufficient tumor response to allow for resection with or without ablation in a small number of patients.
For patients with extensive CRHM, a staged resection-ablation strategy may be appropriate. The optimal initial modality will be dependent on tumor biology, anatomy, and patient condition, as mentioned above. Based on pre- and post-treatment imaging the resection-ablation strategy may need to be adjusted to accommodate tumor response or lack thereof. Interval imaging following the initial intervention may demonstrate that the patient’s disease is not ultimately resectable and therefore the patient should proceed to systemic therapy, palliative thermal tumor ablation, or potential enrollment into a clinical trial. Conversely, if after the initial liver-directed procedure, subsequent imaging supports that complete CRHM eradication can be achieved either by resection or by combined resection + TTA, then the treatment plan would thus proceed, accordingly. We now consider several common scenarios in which thermal tumor ablation may be appropriate.
Synchronous presentation of the colorectal primary and single or low volume CRHM
As the frequency of laparoscopic colorectal resections continues to increase, or in open cases with adequate exposure, the use of TTA in the same setting is reasonable if clearance of the liver disease would require a major (lobectomy or greater), unplanned hepatic resection or if patient can not tolerate simultaneous primary tumor and hepatic resection. Caveats to this approach, and for the initial combined resection-ablation strategy, are that for conventional thermal tumor ablation, lesions should be less than 4 cm. If bilobar disease is present, then ablation should not compromise inflow or outflow tracts as a consequence of hepatic swelling, as this may compromise future liver resection. Utilization of intra-operative ultrasound is employed both for targeting TTA, avoiding treatment failure, and protecting vital intrahepatic structures. Use of ablation for management of synchronous CRHM during primary tumor resection may limit the morbidity when compared to simultaneous colorectal and liver resections, although both can be performed safely in selected patients (46). It is worth noting that in the setting of CRHM, the need for resection of the primary tumor in the absence of over bleeding or obstruction may not be necessary and could delay more pressing issues including the management of CRHM or extrahepatic disease (47,48).
Bilobar CRHM with the ability to render an appropriate volume of liver free of disease, upon which the future hepatic remnant can be based
This is perhaps the most common clinical scenario in which ablation complements resection. Any staged treatment plan will ultimately require that after planned interventions, a portion of liver remains with uncompromised inflow and outflow, ideally completely clear of disease. Although not the focus of this review, portal vein embolization (PVE) has enabled the hepatic surgeon to offer staged approaches to a greater number of CRHM patients through the optimization of future liver remnant volume (49,50). Consider the patient with right hepatic lobe dominant disease and an isolated CRHM in segment III. The authors would advocate that this patient should proceed to undergo a partial left hepatic lobectomy (laparoscopic approach preferred) and thermal tumor ablation of any lesion at risk of crossing the main portal scissurae as defined by the middle hepatic vein. Subsequently, the right portal vein is embolized to induce left liver hypertrophy in anticipation of a right formal hepatectomy.
In a patient with more extensive, bilobar CRHM in segments II/III, IV, VIII (dome), and VI lesion, several approaches are possible. The optimal strategy would be based on the relationship of the tumors to major vascular and biliary structures, in addition to optimizing liver remnant volume. One approach would be to perform a formal left hemi-hepatectomy (clearing II/III and IV-A) and non-anatomic resections of the segment VIII and segment VI lesions. Another approach, again depending on proximity to vital intrahepatic structures, would be to use thermal ablation for the segment VIII lesion and resect the others as previously proposed. For a lesion <3 cm and away from potential heat sinks, thermal ablation offers a reasonable alternative to resection when patient and/or tumor factors prohibit surgical extirpation (51,52). In this scenario, thermal ablation allows for the staging of liver-directed therapies in selected patients, which may mitigate some of the risk(s) associated with major hepatectomies and maximize the preservation of functioning liver parenchyma.
Intra-operatively identified additional CRHM disease
Intra-operatively identified CRHM not detected by preoperative imaging are rare with modern imaging techniques and occur in 10-12% of patients (53-55). In general, these are sub-centimeter sized lesions and are identified by intra-operative ultrasound examination or palpation. When these lesions are identified, and not otherwise included in the planned resection, MWA or RFA offer the opportunity to treat the lesions if it is not possible to safely include them in a resection. Again, based on the principle, that for a lesion <3 cm and away from potential heat sinks, TTA is a valuable option in patients who are not suitable candidates for complete CRHM resection (51,52).
Single or low volume CRHM with limited resectable pulmonary metastases
For CRHM patients with extrahepatic disease in the lungs, our willingness to perform major hepatic resections is tempered by the aggressive tumor biology or heightened risk for recurrent disease following treatment. As such, for patients with both liver and lung metastases, RFA or MWA for the management of the CRHM is a valuable option if a major hepatectomy would be required to clear the liver of disease. Long-term survival is possible in highly selected patients with limited lung and liver colorectal metastases (56,57). Such management plans are carried out in the context of systemic therapy. Although not addressed in this review, ablative modalities are also employed in the treatment of lung metastases.
Is thermal ablation alone reasonable for unresectable CRHM?
For this scenario to arise, the patient may not have been resectable at presentation, there was insufficient down staging from systemic therapy, and/or initial partial tumor clearance with the intent to return for a second staged operation has failed due to progressive disease. We argue that there is a limited role for TTA in the unresectable patient with liver-only disease.
There is general agreement that systemic chemotherapy +/- biologic agents is the mainstay of therapy for an unresectable patient. Although too complex to be adequately discussed in this article, the various combinations of systemic chemotherapy agents and now the handful of monoclonal antibody therapies offer meaningful response rates. We now consider whether TTA is a useful modality when complete CRHM clearance is not a reasonable goal.
The 10-year Cleveland clinic experience (58) demonstrates that patients undergoing thermal tumor ablation and receiving adjuvant chemotherapy had a survival benefit compared to patients receiving only systemic chemotherapy or importantly, only thermal tumor ablation. The median survival was 28 months in the group receiving both modalities compared to 18-19 months in those treated with only chemotherapy or ablation. As would be expected, survival was significantly correlated with the number of lesions ablated and therefore the extent of intrahepatic disease which likely reflected overall tumor biology. An EORTC study (59) compared systemic chemotherapy (CT) alone to CT plus thermal tumor ablation and demonstrated a significant improvement in median progression free survival with the combined approach (16.8+ CT vs. 9.9 months, P=0.025), although the 30-month overall survival difference was not significant.
As a summary observation, for patients with unresectable CRHM, if thermal tumor ablation can be safely performed, then the addition of TTA to systemic chemotherapy is a reasonable approach to control intrahepatic disease. Interestingly, recent literature suggests that both ablation and systemic agents may improve the host immune response to CRHM, which has been associated with improved survival (60). However, the superior outcomes of patients who received ablation in addition to systemic therapy may be in part dependent on selection of those with more favorable tumor biology.
Should thermal tumor ablation be used in lieu of resection?
This strategy may be applicable in select patients with contraindication to surgical resection in relation to extent of disease or medical co-morbidities. There are limitations to consider for avoiding treatment failure and/or hepatic damage. Initially, the size limit for tumors for RFA was 3cm, however over the last few years with increasingly powerful generators and improved needle configurations the lesion size cutoff has moved to 4cm. The advent of MWA technology has largely removed the theoretical limits of an ablation size, although many lesions larger than 5cm are in close proximity to major portal structures.
Although there have been no prospective randomized trials comparing RFA to resection, nor are there likely to ever be, the currently available data suggest evidence that RFA is an effective modality in the treatment of selected patients with CRHM <3cm in size, who are not suitable candidates for surgical resection. In a study by Berber et al. (61), median overall survival for patients with unresectable CRHM, after laparoscopic RFA, was 28.9 months compared to historical controls with chemotherapy alone (10 to 14 months). In a study by Oshowo et al. (62), who treated patients with solitary CRHM, median survival after liver resection was 41 months compared to 37 months for RFA, while 3-year survival rate was 55.4% for resection compared to 52.6% for RFA, although 3-year follow up is not adequate. In another study (51), Hur et al. demonstrated that in RHM <3 cm, the 5-year survival rates following resection and RFA were similar, including overall (56.1% vs. 55.4%, P=0.451) and local recurrence-free (95.7% vs. 85.6%, P=0.304) survival rates, suggesting that RFA is an acceptable alternative treatment in patients with solitary CRHM smaller than 3 cm who are not candidates for resection. In another study, Otto et al. (63) showed that there is no difference in overall 3-year survival between resection and RFA for early CRHM, even though RFA was associated with higher local tumor recurrence rates and shorter time to progression. In yet another recent study, Kim et al. (52) suggest that RFA may be a safe alternative treatment for solitary CRHM <3 cm, with equivalent outcomes (overall and disease-free survival) compared to resection. These data suggest that RFA represents an effective local treatment for patients who are unsuitable for conventional surgical treatment. However, caution is warranted in using ablation in lieu of resection for patients who are suitable candidates for surgical treatment. Ablation should NOT be seen as a replacement for hepatic resection and does not preclude the need of systemic chemotherapy. Furthermore, the candidates for this specific approach are likely to be few.
Important clinical and technical considerations for thermal tumor ablation
Just as the vast majority of patients with CRHM are not candidates for potentially curative resection, most will also not be candidates for evolving strategies that includes staged hepatic resection with or without tumor ablation, regional infusion therapies, and the preceding approaches in the context of systemic regimens. The evolving field of regional hepatic therapies lacks mature data to guide the approach, such as the optimal sequence of therapies and defining the target patient population that may be most likely to benefit. As such, we put forth a few caveats, which are critical in the treatment planning process for these complex patients.
The most important determinant of outcome for patients with CRHM is the biology or extent of disease. Regardless of the treatment efficacy of any one modality at the local level (liver), the presence of progressive, persistent, or chemotherapy-refractory systemic disease should in most instances preclude the use of resection or thermal tumor ablation. Assuming the conditions described in the previous paragraph are met, the limitations of thermal tumor ablation are straightforward, and by no means complete in the listing that follows: (I) At any given time, there must be sufficient hepatic reserve to ensure adequate function. (II) The use of ablation as a prelude to resection should encompass the principles of known treatment failures, such as heat sinks. Basing a future liver remnant on a portion of liver at high risk for persistent or recurrent tumor in the ablation zone should be avoided. (III) The potential for inadvertent for injury to vital hepatic structures needs to be carefully considered utilizing TTA for CRHM, particularly in the context of a staged approach to ensure adequate inflow and outflow for the liver remnant. (IV) In the setting of chemotherapy-refractory or progressive disease, TTA should be limited to highly selected patients for control of intrahepatic disease causing specific symptoms.
Conclusion
Surgical resection with a negative tumor margin is still the optimal and only potential curative strategy for patients with CRHM. The vast majority of patients with CRHM are not candidates for curative resection, thus many may benefit from the use of adjunct modalities such as TTA alone or in combination with systemic therapy. In well-selected patients with initially unresectable CRHM, a well formulated multidisciplinary plan may include staged liver resection alone or in combination with thermal ablation. In patients who are not surgical candidates and fail to achieve tumor down staging for conversion to resectability, TTA may enable control of intrahepatic disease for control of symptoms as a component of total oncologic care. TTA is a valuable component of the multimodality management of patients with CRHM that complements resection and systemic therapy. A sound understanding of the indications for and limitations of TTA will enable the clinician to appropriately select patients who may benefit from ablation of liver metastases.
Footnote
No potential conflict of interest.
References
- Steele G Jr, Ravikumar TS. Resection of hepatic metastases from colorectal cancer. Biologic perspective. Ann Surg 1989;210:127-138. [PubMed]
- Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol 1997;15:938-946. [PubMed]
- Donadon M, Ribero D, Morris-Stiff G, Abdalla EK, Vauthey JN. New paradigm in the management of liver-only metastases from colorectal cancer. Gastrointest Cancer Res 2007;1:20-27. [PubMed]
- Wagner JS, Adson MA, Van Heerden JA, Adson MH, Ilstrup DM. The natural history of hepatic metastases from colorectal cancer. A comparison with resective treatment. Ann Surg 1984;199:502-508. [PubMed]
- Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 2000;355:1041-1047. [PubMed]
- de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000;18:2938-2947. [PubMed]
- Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004;22:23-30. [PubMed]
- Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol 2007;25:1670-1676. [PubMed]
- Pawlik TM, Abdalla EK, Ellis LM, Vauthey JN, Curley SA. Debunking dogma: surgery for four or more colorectal liver metastases is justified. J Gastrointest Surg 2006;10:240-248. [PubMed]
- Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 2005;241:715-722, discussion 722-724. [PubMed]
- Figueras J, Valls C, Rafecas A, Fabregat J, Ramos E, Jaurrieta E. Resection rate and effect of postoperative chemotherapy on survival after surgery for colorectal liver metastases. Br J Surg 2001;88:980-985. [PubMed]
- Rubbia-Brandt L, Audard V, Sartoretti P, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol 2004;15:460-466. [PubMed]
- Kooby DA, Fong Y, Suriawinata A, et al. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg 2003;7:1034-1044. [PubMed]
- McGahan JP, Brock JM, Tesluk H, Gu WZ, Schneider P, Browning PD. Hepatic ablation with use of radio-frequency electrocautery in the animal model. J Vasc Interv Radiol 1992;3:291-297. [PubMed]
- Poon RT, Ng KK, Lam CM, et al. Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg 2004;239:441-449. [PubMed]
- Amersi FF, McElrath-Garza A, Ahmad A, et al. Long-term survival after radiofrequency ablation of complex unresectable liver tumors. Arch Surg 2006;141:581-587, discussion 587-588. [PubMed]
- de Meijer VE, Verhoef C, Kuiper JW, Alwayn IP, Kazemier G, Ijzermans JN. Radiofrequency ablation in patients with primary and secondary hepatic malignancies. J Gastrointest Surg 2006;10:960-973. [PubMed]
- Eisele RM, Neumann U, Neuhaus P, Schumacher G. Open surgical is superior to percutaneous access for radiofrequency ablation of hepatic metastases. World J Surg 2009;33:804-811. [PubMed]
- Elias D, Sideris L, Pocard M, et al. Incidence of unsuspected and treatable metastatic disease associated with operable colorectal liver metastases discovered only at laparotomy (and not treated when performing percutaneous radiofrequency ablation). Ann Surg Oncol 2005;12:298-302. [PubMed]
- Wood TF, Rose DM, Chung M, Allegra DP, Foshag LJ, Bilchik AJ. Radiofrequency ablation of 231 unresectable hepatic tumors: indications, limitations, and complications. Ann Surg Oncol 2000;7:593-600. [PubMed]
- Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg 2005;242:158-171. [PubMed]
- Gillams AR, Lees WR. Five-year survival following radiofrequency ablation of small, solitary, hepatic colorectal metastases. J Vasc Interv Radiol 2008;19:712-717. [PubMed]
- Lee WS, Yun SH, Chun HK, et al. Clinical outcomes of hepatic resection and radiofrequency ablation in patients with solitary colorectal liver metastasis. J Clin Gastroenterol 2008;42:945-949. [PubMed]
- Kuvshinoff BW, Ota DM. Radiofrequency ablation of liver tumors: influence of technique and tumor size. Surgery 2002;132:605-611, discussion 611-612. [PubMed]
- Bleicher RJ, Allegra DP, Nora DT, Wood TF, Foshag LJ, Bilchik AJ. Radiofrequency ablation in 447 complex unresectable liver tumors: lessons learned. Ann Surg Oncol 2003;10:52-58. [PubMed]
- Liu CH, Arellano RS, Uppot RN, Samir AE, Gervais DA, Mueller PR. Radiofrequency ablation of hepatic tumours: effect of post-ablation margin on local tumour progression. Eur Radiol 2010;20:877-885. [PubMed]
- Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004;239:818-825, discussion 825-827. [PubMed]
- Mulier S, Ni Y, Miao Y, et al. Size and geometry of hepatic radiofrequency lesions. Eur J Surg Oncol 2003;29:867-878. [PubMed]
- Lu DS, Raman SS, Limanond P, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol 2003;14:1267-1274. [PubMed]
- Tungjitkusolmun S, Staelin ST, Haemmerich D, et al. Three-Dimensional finite-element analyses for radio-frequency hepatic tumor ablation. IEEE Trans Biomed Eng 2002;49:3-9. [PubMed]
- Rossi S, Garbagnati F, De Francesco I, et al. Relationship between the shape and size of radiofrequency induced thermal lesions and hepatic vascularization. Tumori 1999;85:128-132. [PubMed]
- Scott DJ, Fleming JB, Watumull LM, Lindberg G, Tesfay ST, Jones DB. The effect of hepatic inflow occlusion on laparoscopic radiofrequency ablation using simulated tumors. Surg Endosc 2002;16:1286-1291. [PubMed]
- Mulier S, Mulier P, Ni Y, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg 2002;89:1206-1222. [PubMed]
- Garrean S, Hering J, Saied A, Helton WS, Espat NJ. Radiofrequency ablation of primary and metastatic liver tumors: a critical review of the literature. Am J Surg 2008;195:508-520. [PubMed]
- Boutros C, Espat NJ. What, how, and when to offer nonresectional therapy for colorectal cancer liver metastases. J Gastrointest Surg 2011;15:420-422. [PubMed]
- Minami Y, Kudo M, Hatanaka K, et al. Radiofrequency ablation guided by contrast harmonic sonography using perfluorocarbon microbubbles (Sonazoid) for hepatic malignancies: an initial experience. Liver Int 2010;30:759-764. [PubMed]
- Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics 2005;25:S69-S83. [PubMed]
- Izzo F. Other thermal ablation techniques: microwave and interstitial laser ablation of liver tumors. Ann Surg Oncol 2003;10:491-497. [PubMed]
- Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee FT Jr. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 2005;236:132-139. [PubMed]
- Garrean S, Hering J, Saied A, et al. Ultrasound monitoring of a novel microwave ablation (MWA) device in porcine liver: lessons learned and phenomena observed on ablative effects near major intrahepatic vessels. J Gastrointest Surg 2009;13:334-340. [PubMed]
- Gravante G, Ong SL, Metcalfe MS, Strickland A, Dennison AR, Lloyd DM. Hepatic microwave ablation: a review of the histological changes following thermal damage. Liver Int 2008;28:911-921. [PubMed]
- Meloni MF, Andreano A, Lava M, et al. Segmental portal vein thrombosis after microwave ablation of liver tumors: Report of two cases. Eur J Radiol Extra 2010;76:e95-e98.
- Meloni MF, Andreano A, Bovo G, et al. Acute portal venous injury after microwave ablation in an in vivo porcine model: a rare possible complication. J Vasc Interv Radiol 2011;22:947-951. [PubMed]
- Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-318, discussion 318-321. [PubMed]
- Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist 2008;13:51-64. [PubMed]
- Martin R, Paty P, Fong Y, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg 2003;197:233-241, discussion 241-242. [PubMed]
- Scoggins CR, Meszoely IM, Blanke CD, Beauchamp RD, Leach SD. Nonoperative management of primary colorectal cancer in patients with stage IV disease. Ann Surg Oncol 1999;6:651-657. [PubMed]
- Poultsides GA, Servais EL, Saltz LB, et al. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol 2009;27:3379-3384. [PubMed]
- Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 1990;107:521-527. [PubMed]
- Azoulay D, Castaing D, Smail A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg 2000;231:480-486. [PubMed]
- Hur H, Ko YT, Min BS, et al. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg 2009;197:728-736. [PubMed]
- Kim KH, Yoon YS, Yu CS, et al. Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases. J Korean Surg Soc 2011;81:25-34. [PubMed]
- Machi J, Isomoto H, Kurohiji T, et al. Accuracy of intraoperative ultrasonography in diagnosing liver metastasis from colorectal cancer: evaluation with postoperative follow-up results. World J Surg 1991;15:551-556, discussion 557. [PubMed]
- Agrawal N, Fowler AL, Thomas MG. The routine use of intra-operative ultrasound in patients with colorectal cancer improves the detection of hepatic metastases. Colorectal Dis 2006;8:192-194. [PubMed]
- Choti MA, Kaloma F, de Oliveira ML, et al. Patient variability in intraoperative ultrasonographic characteristics of colorectal liver metastases. Arch Surg 2008;143:29-34, discussion 35. [PubMed]
- Neeff H, Hörth W, Makowiec F, et al. Outcome after resection of hepatic and pulmonary metastases of colorectal cancer. J Gastrointest Surg 2009;13:1813-1820. [PubMed]
- Brouquet A, Vauthey JN, Contreras CM, et al. Improved survival after resection of liver and lung colorectal metastases compared with liver-only metastases: a study of 112 patients with limited lung metastatic disease. J Am Coll Surg 2011;213:62-69, discussion 69-71. [PubMed]
- Siperstein AE, Berber E, Ballem N, Parikh RT. Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Ann Surg 2007;246:559-565, discussion 565-567. [PubMed]
- Ruers T, Punt CJ, van Coevorden F, et al. Final results of the EORTC intergroup randomized study 40004 (CLOCC) evaluating the benefit of radiofrequency ablation (RFA) combined with chemotherapy for unresectable colorectal liver metastases (CRC LM). ASCO Meeting Abstracts 2010;28:s3526.
- Katz SC, Pillarisetty V, Bamboat ZM, et al. T cell infiltrate predicts long-term survival following resection of colorectal cancer liver metastases. Ann Surg Oncol 2009;16:2524-2530. [PubMed]
- Berber E, Pelley R, Siperstein AE. Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: a prospective study. J Clin Oncol 2005;23:1358-1364. [PubMed]
- Oshowo A, Gillams A, Harrison E, Lees WR, Taylor I. Comparison of resection and radiofrequency ablation for treatment of solitary colorectal liver metastases. Br J Surg 2003;90:1240-1243. [PubMed]
- Otto G, Düber C, Hoppe-Lotichius M, König J, Heise M, Pitton MB. Radiofrequency ablation as first-line treatment in patients with early colorectal liver metastases amenable to surgery. Ann Surg 2010;251:796-803. [PubMed]