Clinical outcomes of endoscopic submucosal tunnel dissection compared with conventional endoscopic submucosal dissection for superficial esophageal cancer: a systematic review and meta-analysis
Introduction
Esophageal cancer is the 7th most common cancers worldwide with significant mortality. In 2018, it is reported to be responsible for 1 in every 20 cancer deaths (1). Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are both effective endoscopic resection methods for limited esophageal cancer. Both methods can achieve similar cure rates as surgical resection in specialized centers (2). Although ESD is superior to EMR in terms of en bloc resection rate, R0 en bloc resection rate, and relapse (3), it still has limitations in case of large superficial esophageal squamous cell neoplasms (SESCNs), in which submucosal injection cannot attain satisfactory lifting effects (4,5).
ESTD was first proposed in 2009 by Linghu et al. (6,7) who successfully achieved en bloc resection of an 8-cm-long circumferential SESCN in a submucosal tunnel. Unlike the conventional ESD procedure, incisions were created at the anal and oral sides of the lesion after submucosal injection in ESTD. In this way, the endoscopic physician can get a better view of the submucosal layer. Two subsequent lateral mucosal incisions were made to complete the ESD procedure. ESTD has advantage in terms of better view of the working field and shorter operation time (8).
Many studies have tried to verify the efficacy of ESTD; however, no meta-analysis has been published until now. Thus, this study aimed to analyze the efficacy of ESTD and compare it with that of conventional ESD methods.
Methods
Our systematic review protocol has been registered in the International Prospective Register of Systematic Reviews (PROSPERO, www.crd.york.ac.uk/prospero/, registration number: CRD42019129500).
Literature search
We searched the databases of PubMed, Cochrane Library, Web of Science, SinoMed, Wanfang, and CNKI from their inception up to February 1, 2019. The following search terms were used: “endoscopic submucosal tunnel dissection” or “ESTD”, “esophageal cancer”, “esophageal lesion”, and “esophageal dysplasia”. Both free terms and MeSH words were included. Citation and references of retrieved studies were also reviewed. Only articles published in English or Chinese were included.
Study selection
Studies that met the following criteria were included: (I) studies involving patients diagnosed with esophageal carcinoma or precancerous lesions based on histology, (II) studies conducted to compare ESTD and ESD for esophageal carcinoma or precancerous lesions, and (III) studies reporting clinical outcomes after ESTD or ESD, including R0 resection rate, en bloc resection rate, and complications. The exclusion criteria were as follows: (I) case reports or reviews, (II) ESTD or ESD performed in pathological types other than esophageal carcinoma or precancerous lesions, (III) studies not published in Chinese or English language, and (IV) studies including fewer than ten patients in each group.
Data extraction and quality evaluation
Two reviewers independently screened the titles and abstracts of articles. The following information was extracted from the articles: authors, year of publication, country or region, study design, number of patients, and clinical outcomes data including operation time, dissection speed, R0 resection rate, en bloc resection rate, postoperative complications, and duration of hospital stay. The Newcastle-Ottawa Quality Assessment Scale (NOS) was used to evaluate the quality of the enrolled studies, and studies with NOS scores >6 points were considered high-quality articles.
Statistical analysis
Weighted mean differences (WMDs) were chosen for operation time in this meta-analysis. Odds ratios (ORs) were chosen for measuring dichotomous variables. Statistical heterogeneity was assessed by applying Q2 tests and the Higgins I2 statistics. A value of P<0.10 or I2>50% indicated statistical significance. In case of statistically insignificant heterogeneity, a fixed-effects model was adopted. Otherwise, a random-effects model was applied. For significant heterogeneity, sensitivity analysis or subgroup analysis were performed to seek for source of heterogeneity. A value of P<0.05 was considered significant. All statistical analyses were performed using Stata software 14 (Stata Corp, College Station, TX, USA). Begg’s test was performed for publication bias based on R0 resection rate.
Results
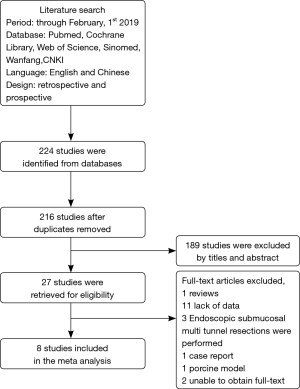
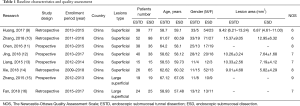
A total of 224 articles were retrieved by literature search (Figure 1), of which only eight articles were finally enrolled after applying the selection criteria, including a total of 625 patients with superficial esophageal cancer. Every study scored 6 or higher in the NOS. The baseline characteristics and quality assessment of the studies are shown in Table 1 (9-16).
Full table
R0 resection
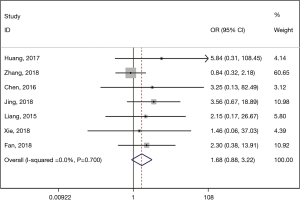
The R0 resection rate was not significantly different between superficial esophageal cancer patients who underwent ESTD and those who underwent ESD (pooled OR: 1.685, 95% CI: 0.881 to 3.222, P=0.115) (Figure 2). A small heterogeneity was detected (I2=0.0%, P=0.700), and a fixed-effects model was used.
En bloc resection
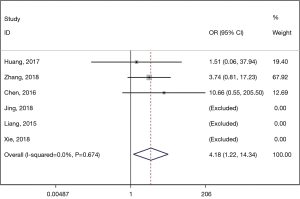
Regarding the en bloc resection rate, six studies reported related data. Three of them achieved 100% en bloc resection rate in both ESTD and ESD groups, so they were excluded in this comparison (17). The results revealed significantly higher en bloc resection rate in the ESTD group than in the ESD group (pooled OR: 4.183, 95% CI: 1.220 to 14.338, P=0.023) (Figure 3). No obvious heterogeneity was found (I2=0.0%, P=0.674).
Postoperative adverse events rate
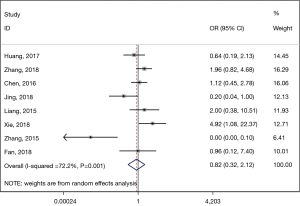
The postoperative adverse event rate was significantly lower in the ESTD group than in the ESD group (pooled OR: 0.824, 95% CI: 0.321 to 2.117, P=0.001) (Figure 4). Significant heterogeneity was detected (I2=72.2%, P=0.001), and a random-effects model was used. Subsequently, sensitivity analysis was performed, which showed that the studies by Zhang et al. (15) in 2015 might have caused the heterogeneity. After excluding these studies, the results revealed a significantly lower heterogeneity (I2=44.7%, P=0.595); however, no difference was shown between the two groups at this time (pooled OR: 1.197, 95% CI: 0.617 to 2.321, P=0.093).
Operation time
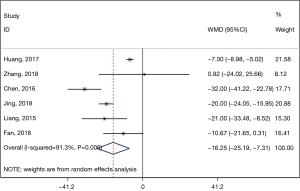
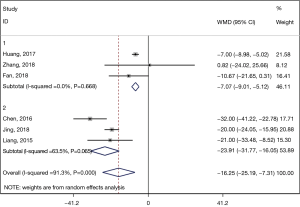
In the comparison of operation time, the ESTD group showed a significantly shorter operation time than the ESD group (pooled WMD: −16.250, 95% CI: −25.186 to −7.313, P=0.000) (Figure 5). Because of the significant heterogeneity (I2=91.3%, P=0.000), we used a random-effects model to perform the analysis. Subsequent subgroup analysis according to the study design revealed a shorter operation time in both prospective (pooled WMD: −7.067, 95% CI: −9.014 to −5.121, P=0.000) studies and retrospective studies (pooled WMD: −23.912, 95% CI: −31.772 to −16.053, P=0.000) (Figure 6). However, the heterogeneity was much lower in the prospective subgroup (I2=0.0%, P=0.668) than in the retrospective group (I2=63.5%, P=0.065).
Recurrence rate
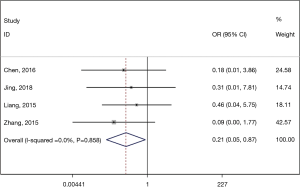
In this comparison, the ESTD group had a significantly lower recurrence rate 1 year after operation than then ESD group (pooled OR: 0.211, 95% CI: 0.052 to 0.865, P=0.031) (Figure 7). No significant heterogeneity was detected (I2=0.0%, P=0.858), and a fixed-effects model was applied.
Publication bias
Begg’s test was performed for publication bias based on R0 resection rate. No publication bias was observed in these analyses (Figure 8, P=0.064).
Discussion
To our knowledge, this is the first meta-analysis comparing ESTD and ESD in treating superficial esophageal cancers, demonstrating the efficacy and safety of ESTD and revealing ESTD as a potentially superior treatment to ESD.
R0 resection, defined as lateral and vertical margins free of carcinoma (18), is a vital parameter closely related to further treatment and recurrence. It should always be considered an important judgment standard when evaluated a new treatment. In our study, The R0 resection rate of ESTD group is comparable with ESD group. En bloc resection is always one of the principles in tumor surgery, and a high en bloc resection rate is one of the reasons why ESD takes the place of EMR and become the primary treatment of early esophageal cancers (19).
Postoperative adverse events may prolong the hospital stay and increase the financial burden of patients and most importantly lower their quality of life. In our study, postoperative adverse events rate was comparable between ESTD group and ESD group. This result is in accordance with former studies about complications (4,8,9,20-22). The ESTD group enjoyed significantly shorter operation time. Arantes et al. believe that standardized ESTD has made esophageal ESD straightforward and less difficult, especially for Western endoscopists (23).
Recurrence was defined as: after surgery of superficial esophageal cancer, the cancer was detected in the follow-up and was confirmed by histopathological method. The mode of recurrence was classified into three patterns: local recurrence was defined as anastomotic recurrence; regional recurrence was defined as that occurring either in the mediastinum or upper abdomen at the site of previous esophageal resection and nodal clearance or in the cervical area where no lymphadenectomy had been performed; and distant recurrence was defined as hematogenous if it developed within a solid organ or within the peritoneal cavity (24). A significantly lower recurrence rate at 1 year was detected in our analysis, which is in accordance with its superior en bloc resection rate. Although our results are satisfying, most studies only included patients with 1-year follow-up. Long-term outcomes still require further randomized controlled trials.
This meta-analysis has several limitations. First, only eight studies were included in the analysis, and all of them were conducted in China. Considering the difference in diagnosis standard, incidence, and treatment guideline, large-scale randomized controlled trials or high-quality comparative studies are required for more reliable and universal data. Second, there was significant clinical heterogeneity in the comparison of operation time and postoperative adverse event rate. The heterogeneity of operation time may be caused by the retrospective nature of several included studies since the I2 decreased significantly after subgroup analysis of retrospective and prospective studies. Various factors including different procedures, equipment, and proficiency levels of physicians can lead to heterogeneity in the rates of postoperative adverse events.
In conclusion, our meta-analysis demonstrates that ESTD is an effective treatment with low recurrence rate for superficial esophageal cancers compared with ESD in terms of en bloc rate, operation time, recurrence rate 1 year after operation with comparable R0 resection rate, postoperative adverse event rate. Further large-scale prospective randomized controlled trials with long-term follow-up, especially in Western countries, are required to confirm our data.
Acknowledgments
I would like to give my sincere gratitude to Dr. Chunxiang Jiang for her support on statistics.
Funding: The study is supported by the Key Research Project of Hunan Province (2018SK21311).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Pech O, Bollschweiler E, Manner H, et al. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett's esophagus at two high-volume centers. Ann Surg 2011;254:67-72. [Crossref] [PubMed]
- Cao Y, Liao C, Tan A, et al. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy 2009;41:751-7. [Crossref] [PubMed]
- Linghu E, Feng X, Wang X, et al. Endoscopic submucosal tunnel dissection for large esophageal neoplastic lesions. Endoscopy 2013;45:60-2. [PubMed]
- Tsao SK, Toyonaga T, Morita Y, et al. Modified fishing-line traction system in endoscopic submucosal dissection of large esophageal tumors. Endoscopy 2011;43 Suppl 2 UCTN:E119.
- Linghu E. Therapeutics of Digestive Endoscopic Tunnel Technique. Berlin: Springer Netherlands, 2014.
- Linghu E, Li H, Huang Q, et al. Using tunnel technology dissecting long circumferential lesions of esophagus. China Continuing Medical Education 2011;3:69-71.
- Pioche M, Mais L, Guillaud O, et al. Endoscopic submucosal tunnel dissection for large esophageal neoplastic lesions. Endoscopy 2013;45:1032-4. [Crossref] [PubMed]
- Huang R, Cai H, Zhao X, et al. Efficacy and safety of endoscopic submucosal tunnel dissection for superficial esophageal squamous cell carcinoma: a propensity score matching analysis. Gastrointest Endosc 2017;86:831-8. [Crossref] [PubMed]
- Zhang W, Zhai Y, Chai N, et al. Endoscopic submucosal tunnel dissection and endoscopic submucosal dissection for large superficial esophageal squamous cell neoplasm: efficacy and safety study to guide future practice. Surg Endosc 2018;32:2814-21. [Crossref] [PubMed]
- Chen L, Wang L, Gao S, et al. Effects of endoscopic submucosal tunnel dissection for esophageal superficial neoplasms. Chinese Journal of Laparoscopic Surgery 2016;9:243-7. (Electronic Edition).
- Jing X, Long X. Comparative Analysis between Endoscopic Submucosal Dissection and Endoscopic Submucosal Tunnel Dissection. Medical & Pharmaceutical Journal of Chinese People’s Liberation Army 2018;30:16-9.
- Wei Liang, Xu Lixia, Deng Wanyin, et al. Clinical Value of Endoscopic Submucosal Tunnel Dissection and Endoscopic Submucosal Dissection for Early Esophageal Cancer and Precancerous Lesions. Journal of Fujian Medical University 2015;49:96-100.
- Xie X. Modified ESD and conventional ESD treatment comparison of surgical efficiency in early esophageal cancer and precancerous lesions [master]. Lanzhou University; 2018.
- Zhang XW, Feng KX, Zhu JQ. Research on effect of endoscopic submucosal tunnel dissection on patients with large esophageal superficial neoplasms. China Medical Equipment 2015;12:125-7.
- Fan HH. Efficacy and safety of conventional endoscopic submucosal dissection and endoscopic submucosal tunnel dissection in the treatment of large esophageal mucosal lesions. Journal of Snake 2018;30:460-2.
- Pittler M. Systematic Reviews in Health Care: Meta-analysis in Context. Focus Altern Complement Ther 2002;7:206-7. [Crossref]
- Nagata K, Shimizu M. Pathological evaluation of gastrointestinal endoscopic submucosal dissection materials based on Japanese guidelines. World J Gastrointest Endosc 2012;4:489-99. [Crossref] [PubMed]
- Ishihara R, Iishi H, Uedo N, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc 2008;68:1066-72. [Crossref] [PubMed]
- Ye LP, Zheng HH, Mao XL, et al. Complete circular endoscopic resection using submucosal tunnel technique combined with esophageal stent placement for circumferential superficial esophageal lesions. Surg Endosc 2016;30:1078-85. [Crossref] [PubMed]
- Zhai YQ, Li HK, Linghu EQ. Endoscopic submucosal tunnel dissection for large superficial esophageal squamous cell neoplasms. World J Gastroenterol 2016;22:435-45. [Crossref] [PubMed]
- Zhang W, Zhai Y, Chai N, et al. Single- and double-tunnel endoscopic submucosal tunnel dissection for large superficial esophageal squamous cell neoplasms. Endoscopy 2018;50:505-10. [Crossref] [PubMed]
- Arantes V, Albuquerque W, Freitas Dias CA, et al. Standardized endoscopic submucosal tunnel dissection for management of early esophageal tumors (with video). Gastrointest Endosc 2013;78:946-52. [Crossref] [PubMed]
- Mariette C, Balon JM, Piessen G, et al. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer 2003;97:1616-23. [Crossref] [PubMed]