First line nab-paclitaxel plus gemcitabine in elderly metastatic pancreatic patients: a good choice beyond age
Introduction
Pancreatic cancer (PC) is one of the most lethal malignant tumors across the world, including 55,000 new cases and 44,000 deaths estimated in 2018 (1). The risk of developing PC increases with age: the median age at diagnosis is 71 years old (2) with more than 70% of patients (pts) aged between 55 and 84 years. Life expectancy is poor in case of metastatic disease, with a 5-year survival of 2.6% in this setting. However, elderly pts seems to have a worse overall survival (OS) than younger ones (1).
In metastatic setting, chemotherapy remains a palliative approach, even if the recent development of new combination regimens, such as FOLFIRINOX (3) and nab-paclitaxel plus gemcitabine (4) has significantly improved the outcomes of these pts. Based on these results, FOLFIRINOX or nab-paclitaxel plus gemcitabine represent the standard of care in the first line treatment for pts affected by metastatic PC (mPC) with good performance status (PS). However, despite the high incidence of PC in the elderly, this subgroup is commonly underrepresented in clinical trials and very little data are available regarding the management of these pts. In fact, they often receive no therapy due to a concern for treatment related toxicities and the multidisciplinary diagnostic assessment should be mandatory in order to choose the best treatment for these pts. Indeed, although single agent chemotherapy has shown clinical benefit compared to best supportive care alone (5), retrospective studies demonstrate that elderly pts are treated less aggressively and receive chemotherapy less frequently than youngers (6), that could explain in part the lower OS in the older.
The phase III MPACT trial has shown that nab-paclitaxel plus gemcitabine significantly improves OS and progression free survival (PFS) compared to single-agent gemcitabine, but also in this case few data are available in elderly pts. Although this trial did not provide an age cut off in its exclusion criteria, only 42% were 65 years old or older, and 10% of pts were more than 75 years old (4).
Based on the limited data available in literature about the use of nab-paclitaxel plus gemcitabine as first-line treatment for elderly pts affected by mPC, our study aims to show whether standard treatment can be feasible in mPC geriatric population.
Methods
Patients
We retrospectively collected the data of pts ≥65 years old who received nab-paclitaxel plus gemcitabine as first-line chemotherapy for mPC at four European sites (three Italian centers: Division of Medical Oncology of the University of Campania “Luigi Vanvitelli” in Naples, Hospital “A. Cardarelli” in Naples and “Sacro Cuore di Gesù, Fatebenefratelli” in Benevento; one Spanish center: “Consorcio Hospital General Universitario de Valencia”). We used 65 years old as cut off, because the incidence of geriatric problems increases after this period in the oncologic population, according to the literature (7).
Pts with histologically confirmed pancreatic adenocarcinoma, receiving first-line chemotherapy with nab-paclitaxel and gemcitabine, were considered eligible for our analysis. Moreover, we collected the data of pts that received a prior adjuvant treatment with gemcitabine if this was completed at least 6 months before the relapse of disease. Pts who received previous anticancer treatments in a first-line setting, randomized in clinical trial or with incomplete laboratory reports were excluded from this study. The institutional board at the center approved the protocol and the study was done in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All pts provided written informed consent (at the time of therapy) about the use of their data for future medical research.
The following clinical and pathological variables were recorded from pts’ history before starting first-line chemotherapy: gender, age at diagnosis, evaluation of comorbidities and usual medications, Eastern Cooperative Oncology Group PS [ECOG PS criteria on a 5-point scale, with 0 indicating no symptoms and higher numbers indicating greater disability up to 5 for dead (8)], tumor site, metastatic status, sites of metastasis (liver/lung/peritoneum/other), presence of biliary stent, tumor marker (carbohydrate antigen 19-9: CA 19.9) and albumin levels.
Treatment and follow-up
All pts treated with at least one cycle of nab-paclitaxel plus gemcitabine were included in the analysis. Nab-paclitaxel 125 mg/m2, followed by gemcitabine 1,000 mg/m2, was administered intravenously on days 1, 8 and 15 every 4 weeks until progression of disease (PD), unacceptable toxicity or patient refusal. Antiemetic prophylaxis with serotonin type 3 receptor antagonists plus dexamethasone was used in all pts. Recombinant human granulocyte colony-stimulating factor (G-CSF) and erythropoietin were administered as needed by physician. Dose reductions were applied in cases of grade 3/4 toxicities and in case of persistent grade 2 toxicities at discretion of the physician. Dose reductions were based on adverse events (AEs) that were graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03Jeny (9). Treatment was temporarily suspended in cases of grade 3/4 hematological toxicity or grade 2 or higher nonhematological toxicity, according to clinical practice. The doses that were not administered were considered lost and were not re-administered. Once the toxicity level was reduced to grade 1 or below, chemotherapy was continued at a lower dose. The treatment was suspended if the pts experienced further toxicity. Dose re-escalation was not applied in this setting. An assessment with tumor marker levels and total body computed tomography (CT) scan was performed every 2 months in all pts to evaluate the response, according to the criteria of Response Evaluation Criteria in Solid tumors (RECIST) 1.1 (10). Magnetic resonance imaging (MRI), scintigraphic bone scan, brain CT scan or 18-fluorodeoxyglucose positron emission tomography (18-FDG PET) were performed as needed in addition to CT evaluation in controversial cases. Moreover, the changing in autonomy and toxicities were evaluated during all the treatment period before administering each dose.
Pts who interrupted the treatment but did not show PD were followed up with a total body CT scan and tumor marker determination every 2 months until evidence of PD or death for any reason. As in the treatment period, MRI, scintigraphic bone scan, brain CT scan or 18-FDG PET were performed as needed to complete the CT evaluation in controversial cases in the follow-up period as well. The response was evaluated according to RECIST 1.1. criteria (10).
Statistical analysis
Survival distribution was estimated by the Kaplan-Meier method with 95% confidence interval (CI). The differences in survival according to clinical parameters or treatment were evaluated by the log-rank test and described by the Kaplan-Meier method. For the final analysis, the survival status of all pts was updated within 1 month before the data cut-off date. Cox proportional-hazards model was applied to the multivariate survival analysis. All the significant variables in the univariate model were used to build the multivariate model of survival. SPSS software (version 21.00; SPSS, Chicago, IL, USA) was used for statistical analysis. A significant level of 0.05 was chosen to assess the statistical significance.
Results
Patients’ characteristics
We revised the data of 64 elderly pts (≥65 years old) affected by mPC and treated at 4 academic and community hospitals involved in this study from October 2014 to December 2017. The last follow-up time was January 08, 2018.
The main demographic and baseline characteristics of pts are shown in Table 1. The median age was 69.5 years with a range of 65–80; males: 23 (35.9%); ECOG PS 2: 9 (14.1%); primary location: head 33 (51.6%); biliary stent: 18 (28.1%); previous surgery: 16 (25.0%); adjuvant chemotherapy: 9 (14.1%). All 64 pts were assessable for toxicity, survival and radiological response using RECIST 1.1 criteria (10).
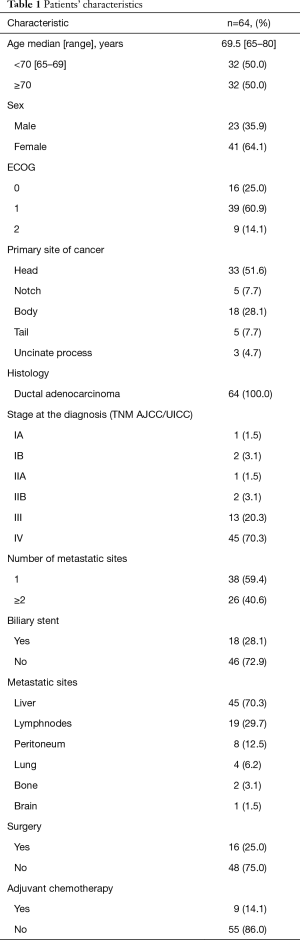
Full table
Toxicities
A median of 5 cycles of chemotherapy were administrated (range, 1–12). Haematological and non haematological toxicities were collected from pts’ history and are presented in Table 2. No grade 4 AEs according to the CTCAE 4.03 (9) was recorded. Fifteen pts (23.4%) did not reported any toxicity. The most frequent AEs were alopecia (60.9%), anaemia (31.2%), neutropenia (20.3%), fatigue (18.7%), nausea (12.5%), hypertransaminasemia (12.5%) and neuropathy (10.9%). Concerning treatment administration, during the overall treatment period, 28 pts received a reduced dose (43.8%) and 27 pts (42.2%) had a delay in dose administration (these doses were not re-administered). G-CSF was administered in 11 pts (17.2%), whereas erythropoietin was used in 13 pts (20.3%). Treatment was stopped for 13 pts (20.3%) due to PD, whereas no pts stopped treatment due to unacceptable toxicity or refusal.
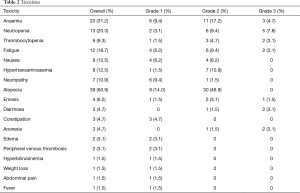
Full table
Objective tumor response and survival
The assessment of the best tumor response according to RECIST 1.1 criteria (10) showed no complete responses, partial response (PR) in 26.6% (n=17), stable disease (SD) in 31.2% (n=20) and PD in 20.3% (n=13) of pts. The evaluation of best response was not applicable in 21.8% (n=14) of pts due to the ongoing treatment without assessment at the moment of data cut-off. Therefore, the objective response rate (ORR) was 26.6% and the disease control rate (DCR) was 57.8% (Table 3).
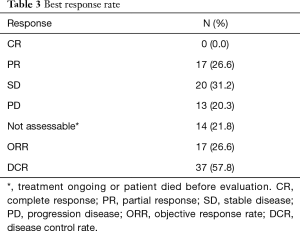
Full table
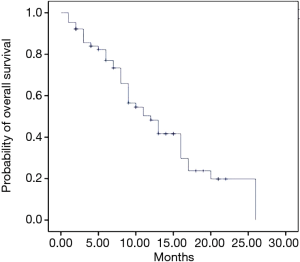
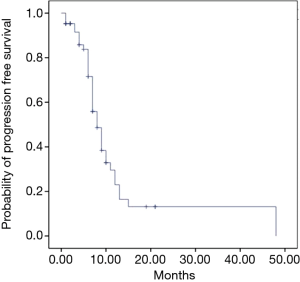
After a median follow-up of 18 months (range, 13.4–22.6 months), median OS was 12 months (95% CI: 8.4–15.6 months) (Figure 1) and median PFS was 8 months (95% CI: 6.3–9.6 months) (Figure 2). The majority of pts died for the disease (62.5%, n=40), whereas 24 pts (37.5%) are still alive at the time of data cut-off. Regarding PFS, 26 pts (40.6%) did not progress (died without PD or treatment still ongoing), whereas 38 pts (59.4%) showed PD. Of these, 24 pts (37.5%) received at least one cycle of second line treatment, according to the following schedules: Capecitabine plus oxaliplatin (Xelox: 18.7%); capecitabine (6.2%); 5-fluorouracil, oxaliplatin and irinotecan (Folfirinox: 3.2%); 5fluorouracil plus irinotecan (Folfiri: 3.1%); Liposomal irinotecan (Naliri) plus 5-fluorouracil [after the publication of NAPOLI-1 trial results (11) as compassionate use allowed only in the University of Campania “Luigi Vanvitelli”]: gemcitabine plus oxaliplatin (1.5%) and 5-fluorouracil plus oxaliplatin (Folfox: 1.5%).
In the univariate analysis for OS, only ECOG PS showed to be an independent prognostic factor, whereas gender, age, site of primary tumor, number and sites of metastasis, presence of biliary stent and surgery were not linked to survival (Table 4). None factors reported in the analysis for OS showed to be related to PFS at univariate analysis.
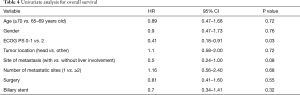
Full table
Discussion
This retrospective analysis aims to show whether standard treatment can be feasible in a geriatric population with mPC considered the lack of evidence in the elderly treated in the everyday clinical practice and the historical disposition to a less aggressive approach in these frail pts.
The trend of aging of the global population and the increasing availability of novel drugs necessitate a deepening on the correct approach for this setting of pts, suggesting that age alone should not be considered a limiting factor for treat the elderly. Assuming that a comprehensive geriatric assessment (CGA) (12) should always be done and that older population has high incidence of comorbidities, in general, for metastatic disease, the prognosis of cancer often supersedes that of any other geriatric disorder leading to a careful consideration of risk/benefit ratio of the treatment. However, to date it is not clear which geriatric functional test should be performed in these pts. Recently, the GrantPax multicenter phase IV trial (13) prospectly evaluated Nab-paclitaxel plus gemcitabine in elderly pts using CGA [activities of daily living (ADL), instrumental activities of daily living (IADL), ECOG and G8 questionnaire] to choose the appropriate first-line treatment in this population. The authors stratify pts according to their functional status: go-go (functional independent pts without comorbidities), slow go (intermediate) and frail (dependent in activities, with comorbidities or geriatric syndromes) (7,13). Go-go pts received standard treatment, whereas intermediate ones received the standard with dose reduction or monotherapy with gemcitabine and the frails received best supportive care only. The authors concluded that the GrantPax approach could guide the choice of personalized treatment for elderly pts (13).
The feasibility of the nab-paclitaxel plus gemcitabine combination shown in the literature (4) could lead to evaluate this schedule also in the first-line treatment of geriatric population (14). In particular, in our analysis Nab-paclitaxel plus gemcitabine showed a safety and efficacy similar to that of younger pts in the MPACT trial (4), proving that age should not be considered an independent prognostic or limiting factor on itself. However, the MPACT trial lacked a geriatric assessment for elderly pts. In our analyses, no serious lifethreatening AE was recorded and the majority of toxicities were of grade 2 or lower. Global incidence of grade 3 toxicities was 26.5%, whereas no grade 4 toxicity was recorded and no pts stopped treatment due to unacceptable toxicity. Unlike the MPACT trial (4), in our analysis the most common AE was anemia (31.2%); there were acceptable rates of fatigue, nausea and neuropathy and general good feasibility of the study regimen. The use of G-CSF and erythropoietin—overall in 17.2% and 20.3% of pts, respectively—led to a good management of haematologic toxicity in our population. However, their use in a population with high burden of comorbidities such as the old one should be always considered only after careful evaluation and tailored on each patient, since erythropoietin in particular is not recommended in the setting of active cancer therapy based on large literature studies, as reviewed by Debeljak et al. (15). According to this, in fact, erythropoietin could enhance the tumor growth through an antiapoptotic effect as well as lead to thromboembolic complications especially in pts with cardiovascular comorbidities. Therefore, the authors suggest to use of erythropoietin with great caution for anemia in case of palliative treatment, avoiding it in case of curative one.
Regarding the efficacy, the combination of Nab-paclitaxel plus gemcitabine showed a promising profile in our population. In fact, DCR and ORR were 57.8% and 26.6%, respectively, with PR and SD reported in 26.6% and 31.2% of pts. These data are important especially if we considered that there was a dose reduction due to toxicities in 43.8% of pts and a delay in dose administration in 42.2% of pts. However, these data were in line with the percentage previously described in the MPACT trial (4), showing dose reduction or delay in the administration in 41% and 54% of pts, respectively. Moreover, our retrospective analysis showed median OS and PFS of 12 and 8 months, respectively, that were better than the outcomes showed in the few, similar previous experiences reported in the literature [median OS: 10 months reported by Giordano et al. (16) and De Vita et al. (17); 6.3 months reported by Vogl et al. (18)]. In particular, we previously reported the data of 41 pts treated with MPACT schedule (27% ≥70 years old) with a good safety profile (17) as well as Vogl et al. (18), showed in 33 pts with mPC a median age of 70 years old and a feasible profile with a modified schedule (13% grade 3 neutropenia, 17% thrombocytopenia and 7% neuropathy).
Therefore, our recent data suggest that first-line chemotherapy with nab-paclitaxel plus gemcitabine is active also in the elderly or frail pts, even if some dose reductions might be required. These findings are in accord with the recent data in the literature, that are investigating the role of nap-paclitaxel plus gemcitabine with a modified schedule in pts with PS 2 at diagnosis (19,20).
In our analysis, only PS according to ECOG scale showed to be independently related to survival at the univariate analysis, whereas the other clinic-pathological parameters, including the age, did not show a prognostic meaning. Finally, our study has some limitations. First, the analysis was retrospective and this fact could lead to a possible and misunderstood lack of data in some cases. In particular, there is a lack of geriatric assessment, which should be important for a study focusing on older pts. However, even if pts underwent to a geriatric evaluation, the geriatric assessment was not included in the oncologic record and the retrospective collection of data lead to the lack of this important data. Second, we collected the data of 64 pts from four European institutions that could seem a small number of pts for each center during the study timeline. However, it is noteworthy as our population represents in our knowledge one of the largest available geriatric sample analyzed for an active treatment for mPC today, since the only more numerous experiences are not reported as paper in extenso until today (16). On the contrary, we could consider the inclusion of the only elderly population as a strenghtness of our analysis. In fact, there is a very low number of evidences including only geriatric pts treated for mPC, whereas the majority of available data came from subgroup analysis of retrospective data (16-18).
Conclusions
Nab-paclitaxel plus gemcitabine is a feasible combination in geriatric population with manageable toxicity levels. This regimen showed to be effectively administrable in a group of pts who will represent an increasing proportion of population, deserving to be treated as best as possible. Therefore, the role of chemotherapy should always be considered also in old and frail pts after GCA on the base of the good toxicity profile and efficacy expected.
Acknowledgments
We thank all patients and their families for the participation in this study.
Footnote
Conflicts of Interest: A Petrillo: Honoraria from Lilly. V Sforza: honoraria from Celgene for “proyecto enlace” and during that period he collected these data. F Ciardiello: Advisory Boards: Roche, Amgen, Merck, Pfizer, Sanofi, Bayer, Servier, BMS, Celgene, Lilly; Institutional Research Grants: Bayer, Roche, Merck, Amgen, AstraZeneca, Takeda. M Orditura: Honoraria from Italfarmaco, EISAI, epionpharma, Roche. F De Vita: Advisory Boards: Roche, Amgen, Celgene, Lilly. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
- SEER Cancer Stat Facts: Pancreatic Cancer. National Cancer Institute. Bethesda, MD. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [Crossref] [PubMed]
- Aldoss TI, Tashi T, Gonsalves W, et al. Role of chemotherapy in the very elderly patients with metastatic pancreatic cancer — A Veterans Affairs Cancer Registry analysis. J Geriatr Oncol 2011;2:209-14. [Crossref]
- Balducci L, Extermann M. Management of cancer in the older person: a practical approach. Oncologist 2000;5:224-37. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- CTCAE 4.03. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. Published: May 28, 2009 (v4.03: June 14, 2010). National Cancer Institute.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 2016;387:545-57. [Crossref] [PubMed]
- Stuck AE, Siu AL, Wieland GD, et al. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet 1993;342:1032-6. [Crossref] [PubMed]
- Betge J, Chi-Kern J, Schulte N, et al. A multicenter phase 4 geriatric assessment directed trial to evaluate gemcitabine +/- nab-paclitaxel in elderly pancreatic cancer patients (GrantPax). BMC Cancer 2018;18:747. [Crossref] [PubMed]
- Macchini M, Chiaravalli M, Zanon S, et al. Chemotherapy in elderly patients with pancreatic cancer: Efficacy, feasibility and future perspectives. Cancer Treat Rev 2019;72:1-6. [Crossref] [PubMed]
- Debeljak N, Solár P, Sytkowski AJ. Erythropoietin and cancer: the unintended consequences of anemia correction. Front Immunol 2014;5:563. [Crossref] [PubMed]
- Giordano G, Vaccaro V, Lucchini E, et al. Nab-paclitaxel (Nab-P) and gemcitabine (G) as first-line chemotherapy (CT) in advanced pancreatic cancer (APDAC) elderly patients (pts): a Real-life study. J Clin Oncol 2015;33:424. [Crossref]
- De Vita F, Ventriglia J, Febbraro A, et al. NAB-paclitaxel and gemcitabine in metastatic pancreatic ductal adenocarcinoma (PDAC): from clinical trials to clinical practice. BMC Cancer 2016;16:709. [Crossref] [PubMed]
- Vogl U, Vormittag L, Winkler T, et al. Gemcitabine plus nabpaclitaxel in metastatic or locally inoperable pancreatic cancer – a single center experience. Ann Oncol 2015;26:iv52. [Crossref]
- Hidalgo M, Pazo-Cid R, Guillen-Ponce C, et al. A phase I and randomized phase II trial to evaluate the efficacy and safety of nab-paclitaxel (nab-P) in combination with gemcitabine (G) for the treatment of patients with ECOG 2 advanced pancreatic cancer (PDAC) in order to decrease the amount of patients to be considered unfit for this feasible combination. Ann Oncol 2017;28:v209-68. [Crossref]
- Macarulla T, Pazo-Cid R, Guillén-Ponce C, et al. Phase I/II Trial to Evaluate the Efficacy and Safety of Nanoparticle Albumin-Bound Paclitaxel in Combination With Gemcitabine in Patients With Pancreatic Cancer and an ECOG Performance Status of 2. J Clin Oncol 2019;37:230-8. [Crossref] [PubMed]