A critical review of the role of local excision in the treatment of early (T1 and T2) rectal tumors
Introduction
Concurrent with the widespread use of population based screening programs, there are more than 42,000 newly diagnosed cases of rectal cancer each year (1). Radical surgical resection of both the primary tumor and the draining lymph node basin (by either low anterior or abdominoperineal resection) remains the corner stone of curative therapy in rectal cancer of all stages. However, the staging accuracy of endorectal ultrasound (ERUS) and pelvic magnetic resonance imaging (MRI) has led some to question the necessity of a major surgical resection for early T-stage cancers when the entire tumor burden could (theoretically) be resected complete by transanal excision. The optimal treatment of early rectal adenocarcinoma remains debatable, although most surgeons recommend radical resection for T2 lesions.
The surgical approach to rectal cancer has evolved continually over the last 100 years. The trend towards less invasive surgical procedures is clear: from the initial attempts at trans-sacral resection, to the popularization of universal abdominoperineal resection (the Miles procedure), followed by acceptance of low anterior resection (greatly facilitated by surgical stapling technology). Transanal, local excision of early cancers is the logical extension of this trend. At present, radical resection with total mesorectal excision (TME) is the surgical standard of care for rectal cancer. This approach completely removes the primary tumor and draining lymph node basin, allowing accurate and complete pathological staging. Radical resection with TME is also fully curative in patients with node-negative and early T-stage cancers. However, radical resection carries a 2-3% perioperative mortality rate and 20-30% overall complication rate (2). Additionally, long-term complications such as sexual impotence, decreased fecundity in women, alterations in bowel function (e.g., the anterior resection syndrome), and the potential for a permanent ostomy all adversely affect quality of life (2-5).
In contrast, local excision avoids the common complications associated with a major operation allowing for decreased anesthesia, minimal fluid shifts and blood loss in combination with a shorter hospital stay and quicker recovery. But the decreased invasiveness comes at the expense of an oncologically incomplete surgery. Advocates for local excision assert that failure due to occult mesorectal lymph node metastases is potentially treatable with salvage total mesorectal excision. Although current imaging modalities have improved, some patients will not be accurately staged. Only after presenting with a local failure will they receive appropriate adjuvant therapy. For this reason, local excision mandates a strict adherence to an intense post-operative surveillance schedule extending beyond 5 years to detect any recurrence.
Appropriate patient and tumor selection remain a major obstacle to transanal excision of rectal cancer, although advances in understanding tumor biology may improve this process. There are no widely accepted guidelines for utilizing local excision. In general, it is reserved for tumors isolated to the submucosa (T1) that are well to moderately differentiated with low-risk histopathological features. Lymphovascular invasion, perineural invasion, and mucinous components are considered high-risk characteristics, and local excision should be avoided due to an increased rate of lymph node metastasis. In addition, tumors should ideally be <3 cm in size with a clear margin, occupy less than 1/3 of the circumference of the bowel and be mobile/nonfixed (6). Despite these stringent inclusion criteria, local excision continues to be plagued with a high recurrence rate (Table 1). The goal for the treatment of early (T1 and T2) rectal cancer is to optimize oncologic control while minimizing the long-term impact of treatment on quality of life (7). This paper will review the data for both T1 and T2 adenocarcinomas, as well some of the promising surgical and combined modalities for treating these early cancers.
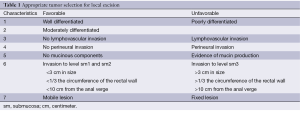
Full table
Transanal excision for T1 adenocarcinoma
Cancer biology differs substantially throughout the lower gastrointestinal tract, with a predisposition for early lymph node spread in the rectum compared to the proximal colon (4). Although limited to the bowel submucosa, T1 rectal cancer has a 13-25% rate of occult lymph node spread compared to only 3-8% in the colon (4,8). While it’s not uncommon to retrieve 1 or 2 lymph nodes along with a full-thickness transanal resection, the nodal basin is not sufficiently staged by local excision alone. Despite lingering concerns about the adequacy of a transanal excision, a paradoxical increase in the use of local excision for T1 tumors occurred in the United States between 1989-2003 (9). Subsequently, several authors have cautioned against local excision, citing excessively high local recurrence rates and worse oncological outcomes (see below), possibly due to lack of rigorous patient and tumor selection.
Most neoplasms less than 10 cm from the anal verge can be resected transanally. Local excision results in a full-thickness specimen including some mesorectal fat. At least 1 cm circumferential mucosal margins should be obtained. The specimen is usually pinned to corkboard or sponge by the surgeon to avoid confusion over orientation and specimen contraction from soaking in Formalin. The defect in the bowel wall is subsequently closed, typically in a transverse manner to prevent restriction of the rectal lumen. Patients perform a full mechanical bowel preparation prior to the surgery, but recovery postoperatively is rapid, with early resumption of regular diet and activity and minimal discomfort.
ERUS and/or pelvic MRI are mandatory for the preoperative staging of rectal cancers. ERUS is more sensitive in distinguishing early bowel invasion of the primary, while MRI is superior at evaluating mesorectal lymph nodes and the circumferential resection margin. The utility of combining ERUS and MRI to direct surgical therapy has also been explored by various investigators (10). Recently though, several studies have reported a significantly lower sensitivity rate (48-54%) of ERUS for detecting early T1 cancer as compared to higher staged lesions (11,12). The success of transanal excision relies on the accuracy of preoperative clinical staging as it fails to address possible occult lymph node metastasis. Presumably the higher regional recurrence rate following local excision is at least in part explained by a failure of preoperative imaging modalities to detect micro-metastatic disease within mesorectal lymph nodes.
The literature on local recurrence rates after transanal excision for T1 rectal cancer is comprised mostly of retrospective studies containing a heterogeneous population of high and low risk lesions (Table 2). Despite the differences between the series, the type of surgery (transanal excision vs. radical resection) remains a constant predictor of local recurrence, with radical resection always maintaining a lower local recurrence rate. The gap between the treatment modalities does narrow though when stratifying tumors by both clinical and pathologic criteria. Blumberg and colleagues demonstrated that excluding high-risk factors (lymphovascular invasion, mucin production, poor differentiation) and applying strict clinical factors (distance from anal verge and size) could decrease the lymph node metastases rate from 16% to 7% (2.3 fold) (5). Kikuchi et al. showed that not all T1 tumors behave in the same manner, and their invasiveness stems from the level of submucosal infiltration. For tumors only slightly invading the submucosa (sm1) there were no nodal metastases observed, as opposed to tumors invading the deepest one-third of the submucosa (sm3) that had a 25% rate of metastases (13). Sm3 depth of invasion has been confirmed by other authors as a contraindication for local excision (8). Greenberg et al. provided long-term follow-up on the prospective CALGB 8984 study of local excision of T1 rectal cancer (14). The authors found a local recurrence rate of only 8% at 7.1 years median follow-up using stringent selection criteria. Others have confirmed that oncological outcomes in prospective series seem improved relative to the larger retrospective reports, reinforcing the importance of strict attention to patient and tumor selection (2).
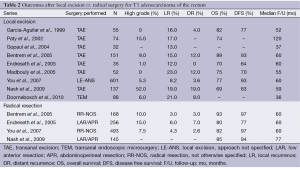
Full table
Although there is an increased local recurrence rate between the surgical modalities, this has failed to translate into a survival benefit. After 5 years, there is an overall survival rate of 70-89% vs. 77-97% and disease free survival rate of 64-93% vs. 80-93% in the transanal excision and radical resection groups, respectively (2,4). Conversely, the similar survival rates may reflect an inadequate follow-up time. During 10 years of follow-up by Nash et al., the authors found a similar overall survival in the first 4 years after diagnosis but an increased rate of cancer-related death between 4-8 years (peak period of cancer recurrence) in the transanal excision group. Only after 9 years did death from other causes dominate in the transanal excision group (12). Patients undergoing local excision must be committed to a long-term follow-up schedule to detect recurrences.
If high-risk features are identified in the original pathologic specimen, an immediate radical resection should be performed. This does not compromise outcomes and has a 94% disease free survival rate at 5 years (15). In this manner, the transanal excision may be viewed as a “large biopsy”, the results of which may direct further immediate surgery. However, the aggressive use of salvage surgery after identifying a local recurrence can still allow for an R0 resection to be accomplished in a majority of cases (77%) (4). With routine post-operative surveillance, the detection rate is up to 88% with proctoscopy and ERUS alone, although most centers also utilize either computed tomography (CT) or MRI (16). Salvage surgery, though, comes with the cost of increased morbidity compared to an initial radical resection and may require multivisceral resection and an ostomy in up to 43% (4). After salvage surgery, the 5-year overall survival is significantly decreased to 43-56.2% compared to those without a recurrence (3,4). The relatively poor outcomes following salvage surgery emphasize the importance of the appropriate initial treatment of early rectal cancer.
Transanal excision for T2 adenocarcinoma
Similar to T1 tumors, there was almost a fifty percent increase across the US between 1989-2003 in the use of local excision to treat T2 rectal cancer (12% to 21%) (7). While local excision is now generally an acceptable treatment of T1 tumors, there is a growing concern about extending its application to T2. Transanal excision of T2 tumors carries a nearly double local recurrence rate compared to T1 lesions, ranging from 13-30% for the more advanced primary lesions (Table 3). The higher local recurrence rate is likely due to the increased occult nodal metastasis rate of 28-38% (17). Conversely, radical resection has only a slightly increased rate of local recurrence at 7.2% compared to that for T1 tumors (9). This finding emphasizes both the staging and therapeutic benefits of total mesorectal excision.
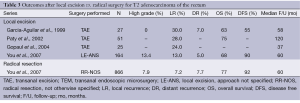
Full table
The increased invasiveness and locoregional metastatic potential of T2 tumors is also reflected in the decreased overall survival, and the difference is increased for patients undergoing local excision as compared to radical resection. In the nationwide cohort study by You et al., there was a significant difference in overall survival (68% vs. 77%, P=0.01) between local excision and radical resection (9). This was strongly impacted, though, by nononcologic factors related to the patient [age (>75) and multiple comorbidities (>2)] rather than the type of surgery. The disease-free survival did not differ (90% vs. 92%, P=0.95) at 5 years, likely due to early death by other non-cancer related causes (9). Given the advanced age and poor health of this study population, they may not have been candidates for a radical resection. Nevertheless, the 90% disease free survival in the radical resection group demonstrates the effectiveness of the procedure in providing a cure.
At present, it seems imprudent to locally excise T2 rectal cancers in fit patients (11). Local excision offers a moderate chance of cure and is reasonable for patients in whom major surgery is contraindicated due to medical comorbidities.
Transanal excision after neoadjuvant chemoradiation
Neoadjuvant chemoradiation has consistently demonstrated the ability to reduce local recurrence rates and downstage primary tumors in select patients with rectal cancers (18,19). This has sparked interest in its application in early rectal cancer (20). Most of the neoadjuvant and adjuvant scheduled in the literature used a similar of radiation dose (50.4-54 Gy), and all chemotherapy regimens are 5-fluoruracil (5-FU) based. As shown in Table 4, tumor downstaging and downsizing has been demonstrated in 51-64% and 26-100% of T2 rectal cancers, respectively (20,21). It is important to note that complete clinical response only translates to a 30-60% pathologically complete response for which there is minimal disease recurrence (20-22). Lezoche et al. reported that overall recurrences occurred primarily in the low response and non-responder groups, at rates of 12% after local excision and 10% after radical resection (21). A more aggressive surgical approach is indicated for these patients, as an incomplete response likewise may exist in the regional lymph nodes (22). Using a neoadjuvant regimen consisting of 4,500 cGy in 25 fractions of radiation over three fields with a boost of 540 cGy to the tumor in conjunction with a continuous infusion of 300 mg m–2 day–1 of 5-FU on days of radiation over a 5-week course, Nair et al. noted that the overall survival was not significantly different between the local excision group and radical resection group (72% vs. 80%, P=0.61) (22). In the transanal excision of T2 rectal cancer, neoadjuvant therapy has shown favorable short-term and similar long-term oncological outcomes to radical resection.
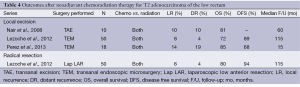
Full table
The improved oncologic benefits of combined chemoradiation therapy do not come without a price. Chemo-radiation increases the rate of post-operative complications; however, most of these are minor complications (91%) that can be managed without additional surgery (20). The most common side effects were gastrointestinal, dermatologic, and hematologic.
The use of neoadjuvant therapy for T2 rectal cancer should not be over utilized, though, as radical surgery alone provides an adequate treatment for T2 N0 disease. It’s role may be to downsize and downstage borderline T2-T3 tumors. Local excision may then be utilized to determine the pathological response to the chemoradiation. If there is only a partial response and tumor still remains, immediate radical resection should be performed. It is the authors’ current practice to determine surgical treatment prior to initiating neoadjuvant therapy.
Transanal endoscopic microsurgery for T1 and T2 adenocarcinoma
Transanal endoscopic microsurgery (TEM) for local excision of rectal adenomas was originally described by Dr. Buess of Germany in 1983. Although the technique and instruments have undergone refinement over the past 30 years, the surgical principles have remained the same. The patient is positioned on the table (lithotomy, prone jackknife, lateral decubitus) such that the tumor is in the posterior position. A specialized set of instruments including a 40 mm rectoscope and laparoscopic style tools are required, although newer minimally invasive equipment can be adapted for transanal use (e.g., transanal minimally invasive surgery). After appropriate insufflation of the rectum, the tumor is visualized and a 1 cm circumferential margin marked with electrocautery. A full thickness excision is then performed, the specimen oriented on the back table and the resulting defect closed transversely with absorbable sutures.
TEM is similar to transanal excision in that patients can expect a short (1-2 day) hospital stay, decreased complications and quicker recovery. The complication rate after TEM is <5% and includes bleeding, rectovaginal fistula, transient incontinence to gas and stool, and transient urinary retention (23). There are several key differences, though, between the two operations. TEM often requires a general anesthetic to perform the procedure, which may be contraindicated in patients with severe cardiopulmonary disease, opposed to spinal or local anesthesia for traditional transanal excision. The superior visualization and instrumentation afforded by TEM relative to traditional transanal excision often permits en bloc specimen removal, thus avoiding piecemeal resection. This allows for an increased rate of R0 resection and a more accurate histological evaluation of the circumferential and deep margin.
TEM is the gold-standard operation for the resection of rectal adenomas, but its use as a curative option for rectal carcinoma is debatable. Despite having a significantly decreased rate of R1 resection between TEM and traditional transanal excision (2% vs. 16%) (24), achieving an R0 resection did not prevent local recurrence (16). Even when stratifying to low-risk T1 tumors, there is still a 17% local recurrence rate after TEM (16). There was no significant difference in the 5-year recurrence rate between T1 and T2 tumors removed by either local excision technique (21% vs. 33%, P=0.07) (24). Due to the high rate of local recurrence in low-risk patients with even an R0 resection, improving criteria for tumor resection by TEM is of major importance.
TEM suffers from the same shortcomings as traditional transanal excision in being unable to adequately stage the pelvis. Using the same post-operative surveillance schedule as transanal excision, most recurrences can be detected early enough to allow for salvage surgery. Short term follow-up after salvage surgery shows a cancer-related survival of 79% at 1 year and 58% at 3 years, which is comparable to transanal excision (16). Between the two local excision modalities, the 5-year disease free survival (85% vs. 70%, P=0.146) and overall survival (80% vs. 66%, P=0.119) were similar across both T1 and T2 lesions (24).
In summary, TEM provides better visualization of the tumor allowing for a more proficient operation to be performed. However, this has not translated to improved local recurrence or overall survival compared to traditional transanal excision. While some authors advocate for TEM as the treatment of choice for local excision, patient and tumor-specific features remain paramount regardless of the surgical approach. Further studies are needed examining the relative effectiveness of TEM compared to traditional transanal excision.
Surveillance following local excision
Following local excision, a long-term surveillance schedule is mandatory to identify recurrences that are potentially resectable and metachronous lesions. Although centers vary slightly in their follow-up regimen, each consists of at least a semiannual history and physical exam, carcinoembryonic antigen (CEA), and proctoscopy in conjunction with annual imaging (CT or MRI) (4,6,11). There has been an increased trend in the combined use of CT/MRI with ERUS postoperatively to increase the sensitivity in detecting locoregional recurrences. It is the authors’ current practice to perform a history and physical examination every 3-6 months for the first 2 years and then annually after. A baseline CEA is obtained prior to surgery and then followed at every appointment. To detect mucosal recurrences, a digital rectal exam and proctoscopy or flexible sigmoidoscopy are performed every 3-6 months for 2 years and then yearly after. This is alternated with ERUS every 6 months to evaluate for lymph node metastases. Finally, a CT or MRI is obtained annually to detect local or distant recurrences. Most surveillance schedules only extend out to five years, but given the propensity for late recurrences, long-term follow-up after local excision should be pursued.
Conclusions
The management of early (T1 and T2) rectal cancer must be individualized to each patient’s expectations of quality and quantity of life. Even in the lowest risk patients, transanal excision is inferior to radical resection from an oncologic standpoint due to inadequate local control and staging of the pelvis leading to an increased local recurrence rate. However, with informed consent, patients may be willing to accept a higher failure rate and an increased post-operative surveillance regimen to preserve a perceived increased quality of life. Accurate and appropriate patient selection for local excision hinges on preoperative imaging techniques and sound histopathology. Local excision remains an acceptable option in well-to-moderately differentiated T1 rectal cancers with favorable histological features, provided the surgeon can obtain clear margins. Future investigations to improve preoperative clinical and pathological staging may improve patient selection and decrease local recurrence.
Acknowledgements
Funding: The authors received no financial support for the research and/or authorship of this article.
Disclosure: The authors declare no conflicts of interest with respect to the authorship and/or publication of this article. The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of the Air Force or Department of Defense.
References
- Stamos MJ, Murrell Z. Management of early rectal T1 and T2 cancers. Clin Cancer Res 2007;13:6885s-9s. [PubMed]
- Endreseth BH, Myrvold HE, Romundstad P, et al. Transanal excision vs. major surgery for T1 rectal cancer. Dis Colon Rectum 2005;48:1380-8. [PubMed]
- Madbouly KM, Remzi FH, Erkek BA, et al. Recurrence after transanal excision of T1 rectal cancer: should we be concerned? Dis Colon Rectum 2005;48:711-9; discussion 719-21. [PubMed]
- Bentrem DJ, Okabe S, Wong WD, et al. T1 adenocarcinoma of the rectum: transanal excision or radical surgery? Ann Surg 2005;242:472-7; discussion 477-9. [PubMed]
- Blumberg D, Paty PB, Guillem JG, et al. All patients with small intramural rectal cancers are at risk for lymph node metastasis. Dis Colon Rectum 1999;42:881-5. [PubMed]
- Network NCC. National Comprehensive Cancer Network: NCCN Clinical Practice Guideline in Oncology: Rectal Cancer- Version 3.2014. Fort Washington, PA: 2014.
- Stitzenberg KB, Sanoff HK, Penn DC, et al. Practice patterns and long-term survival for early-stage rectal cancer. J Clin Oncol 2013;31:4276-82. [PubMed]
- Nascimbeni R, Burgart LJ, Nivatvongs S, et al. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum 2002;45:200-6. [PubMed]
- You YN, Baxter NN, Stewart A, et al. Is the increasing rate of local excision for stage I rectal cancer in the United States justified?: a nationwide cohort study from the National Cancer Database. Ann Surg 2007;245:726-33. [PubMed]
- Glasgow SC. Advancing Dr Wong’s vision for evaluating rectal cancer. Dis Colon Rectum 2013;56:1325-6. [PubMed]
- Garcia-Aguilar J, Mellgren A, Sirivongs P, et al. Local excision of rectal cancer without adjuvant therapy: a word of caution. Ann Surg 2000;231:345-51. [PubMed]
- Nash GM, Weiser MR, Guillem JG, et al. Long-term survival after transanal excision of T1 rectal cancer. Dis Colon Rectum 2009;52:577-82. [PubMed]
- Kikuchi R, Takano M, Takagi K, et al. Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum 1995;38:1286-95. [PubMed]
- Greenberg JA, Shibata D, Herndon JE 2nd, et al. Local excision of distal rectal cancer: an update of cancer and leukemia group B 8984. Dis Colon Rectum 2008;51:1185-91; discussion 1191-4. [PubMed]
- Hahnloser D, Wolff BG, Larson DW, et al. Immediate radical resection after local excision of rectal cancer: an oncologic compromise? Dis Colon Rectum 2005;48:429-37. [PubMed]
- Doornebosch PG, Ferenschild FT, de Wilt JH, et al. Treatment of recurrence after transanal endoscopic microsurgery (TEM) for T1 rectal cancer. Dis Colon Rectum 2010;53:1234-9. [PubMed]
- Gopaul D, Belliveau P, Vuong T, et al. Outcome of local excision of rectal carcinoma. Dis Colon Rectum 2004;47:1780-8. [PubMed]
- Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46. [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [PubMed]
- Garcia-Aguilar J, Shi Q, Thomas CR Jr, et al. A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol 2012;19:384-91. [PubMed]
- Lezoche E, Baldarelli M, Lezoche G, et al. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg 2012;99:1211-8. [PubMed]
- Nair RM, Siegel EM, Chen DT, et al. Long-term results of transanal excision after neoadjuvant chemoradiation for T2 and T3 adenocarcinomas of the rectum. J Gastrointest Surg 2008;12:1797-805; discussion 1805-6.
- Burghardt J, Buess G. Transanal endoscopic microsurgery (TEM): a new technique and development during a time period of 20 years. Surg Technol Int 2005;14:131-7. [PubMed]
- Christoforidis D, Cho HM, Dixon MR, et al. Transanal endoscopic microsurgery versus conventional transanal excision for patients with early rectal cancer. Ann Surg 2009;249:776-82. [PubMed]


