
Preoperative imaging for hepatic resection of colorectal cancer metastasis
Introduction
Colorectal cancer represents the third most common malignancy in the United States afflicting 140,000 patients annually. In a majority of cases, diagnosis is made when cancer is confined to the wall of the colon or rectum and draining lymph nodes. In approximately 20% of patients, however, evidence of cancer spread to distant organs is found concurrent with discovery of the primary lesion. In addition, up to 70% of patients with stage I-III disease initially will develop metastases (stage IV) at some point following diagnosis. The most common site of hematogenous spread is the liver, with 40% of stage IV patients having liver only disease (1). Despite recent advances in chemotherapeutic agents, the prognosis for metastatic colon cancer remains poor, with few patients surviving beyond 5 years. In the past two decades, hepatic metastasectomy has emerged as a promising technique for improving survival in patients with metastatic colon cancer and in some cases providing long-term cure. In a large multi-institutional review of 1568 patients, Nordlinger et al. (2) demonstrated the safety of hepatic metastasectomy with 2.3% operative mortality and actuarial 5-year survival of 28%. The authors identified plurality and size of tumors as predictors of recurrent disease and eventual death. In a retrospective review of 1001 patients undergoing liver resection for colorectal metastases at Memorial Sloan-Kettering Cancer Center, Fong et al. (3) reported similar low operative mortality (2.8%) and 5-year survival of 37% with 22% of patients alive at 10 years. Multivariate analysis revealed node positive primary, presence of extrahepatic disease, CEA >200 ng/mL, >1 tumor, size >5 cm and short disease free interval as predictors for early recurrence and poor overall survival. Using this data, a clinical risk score was created that can help predict who will benefit most from surgical intervention.
Because results from hepatic metastasectomy have been so favorable, a randomized trial assessing its efficacy and safety is impossible at the present time. Therefore, review of retrospective data has been the only means by which to predict those who will recur early and have limited survival. The common poor predictors amongst the various studies have included the size of the primary tumor, presence of multiple hepatic lesions and evidence of extrahepatic disease (2-5). These factors can be best determined preoperatively using cross-sectional imaging. Historically, ultrasonography (US) was the method of choice for identifying hepatic metastases, but advancements in computed tomography (CT), magnetic resonance imaging (MRI) and positron emission tomography (PET) have led to improved detection of occult lesions and better definition of surgical anatomy.
Planning resection
There are many anatomic factors to consider when planning hepatic resection for metastatic disease. When assessing feasibility of resection, it is important to identify the number of segments involved, proximity of lesions to arteries, veins and bile ducts, as well as predict the amount of remnant liver following resection. In most (but not all) patients, involvement of the main portal vein, hepatic artery or common bile duct reflects advanced disease that is not amenable to surgical correction, and alternative options such as palliative chemotherapy are usually recommended. The same is true for multifocal bilobar disease in which resection would leave a diminutive liver remnant insufficient for regeneration and normal hepatic function. Optimal preoperative imaging should readily identify small lesions and provide clear views of the hepatic artery, portal vein and hepatic veins avoiding an unnecessary laparotomy and aborted resection.
While high quality preoperative imaging can help determine resectability, it is equally important in resection planning. With the exception of planned two-stage hepatic resections, the goal of metastasectomy is usually removal of all metastatic lesions while preserving as much unaffected tissue as possible; maintaining segmental arterial blood supply as well as venous and biliary drainage is necessary to achieve this goal. This is rarely an issue for peripherally located lesions, which can typically be removed by wedge resection with little risk to major vessels or bile ducts. However, lesions located deep in the liver parenchyma close to the hilum or hepatic veins require careful attention and planning. For example, a small lesion located close to the origin of the middle hepatic vein may require sacrificing that vessel. Failure to subsequently remove the segments of liver that drain through this vessel may result in significant hepatic congestion and necrosis. This often means performing an extended hepatectomy removing up to 80% of the hepatic parenchyma. If this lesion is not identified preoperatively on appropriate contrast-enhanced imaging and a larger resection is not anticipated, the patient may be left with inadequate liver reserve. When recognized preoperatively, the portal vein supplying the planned resected lobe can be embolized prior to operation, allowing hypertrophy of the contralateral liver, thereby increasing remnant liver volume and reducing the risk of postoperative liver failure.
Identifying lesions within the liver parenchyma can be difficult when extensive hepatic fibrosis or steatosis is present. This is often the case is patients who have received preoperative chemotherapy, particularly oxaliplatin- and irinotecan-based regimens (6). Sinusoidal congestion and fatty replacement can lead to unpredictable alterations in the appearance of the liver resulting in false positive and negative studies. Radiologists reviewing images should be familiar with the extent of pre-imaging chemotherapy to better guide their study evaluation.
Often more than one modality is required to garner the necessary preoperative information. For example, while CT is most commonly used for routine cross-sectional imaging, MRI may be better for identifying occult liver lesions and their proximity to major vessels, and a PET scan better for ruling out extra-hepatic disease. MRI may also be very useful for distinguishing benign tumors from metastases (see below).
Hepatic artery infusion (HAI)
It is well established that neoplasms within the liver receive a majority of their blood supply via the hepatic artery (7). This anatomic fact has been exploited to deliver chemotherapy directly to the hepatic artery by surgically implanted catheters and pumps. Multiple trials of arterial infusion of floxuridine (FUDR) in patients with unresectable hepatic colorectal metastases have been performed with objective response rates of 50-80% (8-11). Indications for HAI therapy have expanded to include adjuvant treatment following hepatic resection (12) and neoadjuvant therapy to allow resectability (13). Although not widely practiced, primarily due to the expertise required for pump placement, maintenance and chemotherapy management, HAI therapy is associated with promising results and is used frequently in some centers.
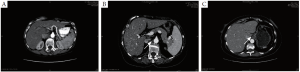
In planning for hepatic artery pump placement, a complete survey of the hepatic arterial anatomy is needed. This is best accomplished with CT angiography with multiplanar and 3D reconstructions. In the normal hepatic arterial anatomy, the common hepatic artery branches from the celiac trunk (Figure 1 A) and gives rise to the gastroduodenal artery (GDA). It is through this branch that the hepatic arterial catheter is placed with the tip at the GDA orifice. This allows for infusion into the proper and subsequently the right and left hepatic arteries. Variations in arterial anatomy occur in approximately 25% of patients (14) and can have a dramatic impact on pump placement and subsequent function. The most common variation is a replaced right or accessory left hepatic artery (Figure 1 B, C) that originates from the superior mesenteric or left gastric artery, respectively. Other anatomic anomalies such as a late takeoff of the right gastric artery are important to recognize as this can lead to inappropriate delivery of toxic chemotherapy to the stomach.
Modalities of hepatic imaging
As previously stated, there are a variety of imaging techniques that can be used to identify lesions within the liver parenchyma, each with their own sensitivity, specificity and resolution. Multiple modalities are often used in the same patient, taking advantage of strengths that one may have over the other. The following is specific information for each imaging technology including strengths and weaknesses:
Computed tomography (CT)
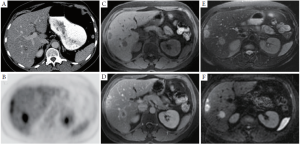
Multidetector computed tomography (MDCT) is routinely used for follow-up of oncology patients, providing robust and rapid imaging of the chest, abdomen and pelvis for detection of liver and extrahepatic metastases. Intravenous iodinated contrast agents are routinely used to improve the detection of liver metastases, which are relatively inconspicuous on non-contrast CT. On routine contrast-enhanced CT (CECT) performed during the portal venous phase, liver metastases are typically hypovascular with variable heterogeneity depending on their size and prior chemotherapy (Figure 2A). Since colorectal liver metastases are hypovascular, the addition of arterial phase imaging generally does not improve their detection (15,16). However, arterial imaging is helpful for pre-surgical or pre-embolization planning.
An important limitation to CECT is in the detection and characterization of subcentimeter liver lesions, which are interpreted as too small to characterize (17). In addition, the development of fatty liver, which is not uncommon following chemotherapy, can further limit the detection of liver metastases. Nevertheless, the spatial resolution of MDCT is superior to MRI and PET, and is especially useful for presurgical planning and identification of aberrant anatomy (18).
Magnetic resonance imaging (MRI)
Magnetic resonance imaging (MRI) examinations generate multiple sequences that highlight different physical properties of water and fat protons in normal and pathologic tissue. Compared to CT, MRI generates lower resolution images that can be prone to artifacts, but benefits from increased soft tissue contrast. In a typical liver MRI, a combination of T1 weighted (T1w), T2 weighted (T2w) and diffusion weighted imaging (DWI) sequences are obtained prior to and following the administration of intravenous gadolinium binding contrast agent (GBCA). Colorectal liver metastases typically demonstrate low signal (hypointensity) compared to liver parenchyma on pre-contrast T1w images, hyperintensity on T2w, and hyperintensity on DWI sequences (Figure 2 C, E, F). Following the administration of intravenous contrast, liver metastases are typically hypovascular with an irregular rim of enhancement (Figure 2 D).
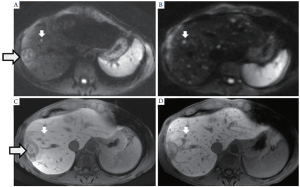
In the last decade, major advances in liver MRI include the development of high resolution volumetric imaging approaching the resolution of MDCT, parallel imaging to reduce scan time, higher static magnetic field strengths (3.0T versus 1.5T), advances in DWI, and hepatocyte-specific contrast agents (19-22). Gd-EOB-DTPA (Eovist or Primovist, Bayer Healthcare Pharmaceuticals, Wayne, NJ) and Gd-BOPTA (MultiHance, Bracco Diagnostics, Princeton, NJ) are two hepatocyte-specific contrast agents that undergo both biliary and renal excretion, as opposed to more traditional GBCAs that only undergo renal excretion, such as Gd-DTPA (Magnevist, Bayer Healthcare Pharmaceutical). With Gd-EOB-DTPA and Gd-BOPTA, delayed hepatobiliary phase T1w images are obtained where normal hepatocytes are markedly hyperintense compared to liver metastases, which do not retain the contrast agent (Figure 3). From our experience, the combination of DWI with Gd-EOB-DTPA MRI yields the highest sensitivity for small liver metastases.
MRI may not be the examination of choice for every patient. Patients with contraindications to MRI (e.g. implantable pacemakers), or unable to tolerate MRI (e.g. due to claustrophobia) would preferably undergo preoperative imaging with CT. Motion related imaging artifacts that can severely dampen the diagnostic quality of MRI will occur in patients who are unable to breath hold for longer than 20 seconds.
Positron emission tomography (PET)
Positron Emission Tomography (PET) is performed to detect the uptake of a glucose analog, 18F-fluorodeoxyglucose (FDG) in hypermetabolic tumors. FDG-PET is now routinely performed in combination with CT, either with or without intravenous contrast, and excels at the detection of colorectal liver and extrahepatic metastases (23). On PET, hypermetabolic liver tumors demonstrate high uptake (Figure 2 B). However, physiologic background liver uptake of FDG in combination with the inherent low resolution of PET can limit the sensitivity for detection of small liver metastases (24). The use of intravenous contrast during the CT portion of the examination is preferred, improving the detection of liver metastases (25).
Comparison between modalities
A recent meta-analysis was performed on prospective studies using CT, MRI, FDG PET (Table 1) between 1990 and January 2010 on metastatic colorectal patients who had not undergone any prior therapy (26). This analysis found large heterogeneity in the methodologies between studies, which is expected when reviewing studies spanning 20 years. The authors concluded that CT generally had the lowest sensitivity, especially for lesions smaller than 10 mm. They also found that MRI had significantly increased sensitivity over the years, especially comparing studies before and after 2004 (from 70% to 85% sensitivity), and recommended MRI as the first line modality, with FDG-PET playing a role for extrahepatic disease detection.

Full table
A recent study comparing multi detector CT, contrast-enhanced US and MRI from Japan found the highest sensitivity for MRI (95%) compared to CT and US (63% and 73% respectively) (27). In this study, MRI was performed with both DWI and delayed hepatobiliary phase imaging using Gd-EOB-DTPA. A limitation in studies comparing imaging modalities for detection of liver metastases is the lack of an absolute reference standard. Histopathologic proof, imaging follow-up and intraoperative ultrasound are often combined as a reference standard, which limits the sensitivity for small metastases and leads to verification bias. In addition, imaging modalities are rarely compared on a lesion-by-lesion basis in the same cohort of patient, leading to selection bias, particularly in favor of MRI and FDG-PET.
In a study comparing MRI with Gd-EOB-DTPA versus PET with CECT in 68 patients undergoing both modalities, MRI demonstrated a higher sensitivity and specificity compared to PET-CECT, especially for lesions smaller than 1 cm (28). A similar study comparing MRI with Gd-EOB-DTPA versus PET-CT (without contrast) also showed a higher sensitivity for MRI (29). These studies reflect our own institutional experience: CT, FDG-PET and MRI have a comparable sensitivity for detection of large liver metastases (Figure 1). However, MRI excels at detection of subcentimeter liver metastases compared to CT and FDG-PET, especially with the combination of DWI and hepatocyte-specific contrast agents (Figure 2).
Volumetrics
One of the most important factors to consider when planning liver resection is the amount of functional parenchyma that will remain after surgery. This is a product of the preserved parenchymal volume and the overall hepatic function. A cursory estimation of liver function can be assessed preoperatively by measurement of hepatic synthetic capability such as production of albumin or clotting factors or its ability to clear bilirubin from the blood. If a more quantitative determination of liver function is needed, a MEGX test can be performed, which evaluates the liver’s ability to convert lidocaine to its metabolite, monoethylglycinexylidide (30). Alternatively, an indocyanine green (ICG) clearance test may be used, which measures the rate of removal of a hepatically excreted dye from the bloodstream (31). This may be particularly important in patients who have been treated with hepatotoxic chemotherapy preoperatively. To estimate the volume of remnant liver following resection, volumetrics is used. First described by Heymsfield (32) in the late 1970s, CT volumetrics uses multiple axial cross-sectional images to recreate the three- dimensional structure of the liver. Using a two-dimensional image from the CT, the outline of the liver is manually traced using appropriate software. This is repeated every 5-10 mm until the entire volume of the liver is calculated. Tumors, cysts and prior ablation cavities should be excluded as these do not significantly contribute to hepatic function. Next, this process is repeated, but this time the outline of the proposed liver remnant is drawn. Typically the venous phase of the CT scan is used for volumetric analysis so that the segmental liver anatomy can be readily identified. The quotient of the calculated remnant and total liver volumes provides the future liver remnant volume, which represents the percentage of hepatic parenchyma remaining. There are currently multiple computer based programs that have automated this process allowing for 3D views of the liver, resection plane and remnant segment (Figure 4).
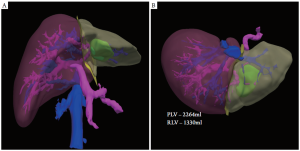
Multiple studies have demonstrated that CT-based volumetric measurement results in a reliable estimation of remnant liver volume with very little interobserver variability (33-35). While no data exist regarding the minimum amount of remnant liver following resection, most agree that 25-30% and 40% of the preoperative volume should be preserved for those with normal and abnormal parenchyma (ie, fibrosis, cirrhosis, steatosis due to preoperative chemotherapy, etc), respectively.
Conclusion
With the increased use of hepatic metastasectomy comes a need for improved imaging techniques to better identify and characterize extent of disease within the liver and elsewhere. While technologic advancements have led to unprecedented image quality and clarity, this does not replace the need for a dedicated, competent radiologist with experience in hepatic imaging. The goals of preoperative imaging should be identification of intra- and extrahepatic disease, relevant liver anatomy and remnant liver volume. Because no one radiologic modality is sufficient to achieve all of these goals, some combination of imaging techniques is needed. At our institution, surveillance and staging imaging is performed with contrast-enhanced CT scans for ease of acquisition and relative low cost. If indeterminate lesions are identified in the liver, MRI may be used to better characterize these lesions and relevant anatomy, as well as rule out the presence of occult disease. MRI is particularly useful for assessing lesions in the presence of steatosis, a common finding after extensive chemotherapy treatment. PET scan is often performed to identify extrahepatic disease and occasionally to better characterize marginal liver lesions. If hepatic artery infusion pump placement is considered, CT angiography with 3D reconstruction is obtained to identify appropriate arterial anatomy. High quality preoperative imaging also allows calculation of the future liver remnant volumes, which is important when extended resections are planned, particularly in the setting of underlying hepatic parenchymal disease. The authors find it particularly helpful to present cases and images at a multidisciplinary tumor board where radiologists, surgeons, oncologists and other physicians can openly discuss findings and prepare the appropriate treatment plan.
Footnote
No potential conflict of interest.
References
- Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 2006;244:254-259. [PubMed]
- Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 1996;77:1254-1262. [PubMed]
- Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-318, discussion 318-321. [PubMed]
- Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist 2008;13:51-64. [PubMed]
- Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg 2007;11:1057-1077. [PubMed]
- Bilchik AJ, Poston G, Curley SA, et al. Neoadjuvant chemotherapy for metastatic colon cancer: a cautionary note. J Clin Oncol 2005;23:9073-9078. [PubMed]
- Sigurdson ER, Ridge JA, Kemeny N, Daly JM. Tumor and liver drug uptake following hepatic artery and portal vein infusion. J Clin Oncol 1987;5:1836-1840. [PubMed]
- Kemeny N, Daly J, Reichman B, Geller N, Botet J, Oderman P. Intrahepatic or systemic infusion of fluorodeoxyuridine in patients with liver metastases from colorectal carcinoma. A randomized trial. Ann Intern Med 1987;107:459-465. [PubMed]
- Kemeny N, Daly J. Preliminary results of a randomized study of intrahepatic infusion versus systemic infusion of 5-fluoro-2’-deoxyuridine for metastatic colorectal carcinoma. Recent Results Cancer Res 1986;100:171-178. [PubMed]
- Kemeny N, Conti JA, Cohen A, et al. Phase II study of hepatic arterial floxuridine, leucovorin, and dexamethasone for unresectable liver metastases from colorectal carcinoma. J Clin Oncol 1994;12:2288-2295. [PubMed]
- Kemeny NE, Jarnagin WR, Capanu M, et al. Randomized phase II trial of adjuvant hepatic arterial infusion and systemic chemotherapy with or without bevacizumab in patients with resected hepatic metastases from colorectal cancer. J Clin Oncol 2011;29:884-889. [PubMed]
- Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med 1999;341:2039-2048. [PubMed]
- Kemeny NE, Melendez FD, Capanu M, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol 2009;27:3465-3471. [PubMed]
- Hiatt JR, Gabbay J, Busuttil RW. Surgical anatomy of the hepatic arteries in 1000 cases. Ann Surg 1994;220:50-52. [PubMed]
- Soyer P, Poccard M, Boudiaf M, et al. Detection of hypovascular hepatic metastases at triple-phase helical CT: sensitivity of phases and comparison with surgical and histopathologic findings. Radiology 2004;231:413-420. [PubMed]
- Wicherts DA, de Haas RJ, van Kessel CS, et al. Incremental value of arterial and equilibrium phase compared to hepatic venous phase CT in the preoperative staging of colorectal liver metastases: an evaluation with different reference standards. Eur J Radiol 2011;77:305-311. [PubMed]
- Khalil HI, Patterson SA, Panicek DM. Hepatic lesions deemed too small to characterize at CT: prevalence and importance in women with breast cancer. Radiology 2005;235:872-878. [PubMed]
- Sahani DV, Krishnamurthy SK, Kalva S, et al. Multidetector-row computed tomography angiography for planning intra-arterial chemotherapy pump placement in patients with colorectal metastases to the liver. J Comput Assist Tomogr 2004;28:478-484. [PubMed]
- Erturk SM, Alberich-Bayarri A, Herrmann KA, Marti-Bonmati L, Ros PR. Use of 3.0-T MR imaging for evaluation of the abdomen. Radiographics 2009;29:1547-1563. [PubMed]
- Taouli B, Koh DM. Diffusion-weighted MR imaging of the liver. Radiology 2010;254:47-66. [PubMed]
- Seale MK, Catalano OA, Saini S, Hahn PF, Sahani DV. Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics 2009;29:1725-1748. [PubMed]
- Goodwin MD, Dobson JE, Sirlin CB, Lim BG, Stella DL. Diagnostic challenges and pitfalls in MR imaging with hepatocyte-specific contrast agents. Radiographics 2011;31:1547-1568. [PubMed]
- Sacks A, Peller PJ, Surasi DS, Chatburn L, Mercier G, Subramaniam RM. Value of PET/CT in the management of liver metastases, part 1. AJR Am J Roentgenol 2011;197:W256-9. [PubMed]
- Kong G, Jackson C, Koh DM, et al. The use of 18F-FDG PET/CT in colorectal liver metastases--comparison with CT and liver MRI. Eur J Nucl Med Mol Imaging 2008;35:1323-1329. [PubMed]
- Badiee S, Franc BL, Webb EM, et al. Role of IV iodinated contrast material in 18F-FDG PET/CT of liver metastases. AJR Am J Roentgenol 2008;191:1436-1439. [PubMed]
- Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology 2010;257:674-684. [PubMed]
- Muhi A, Ichikawa T, Motosugi U, et al. Diagnosis of colorectal hepatic metastases: comparison of contrast-enhanced CT, contrast-enhanced US, superparamagnetic iron oxide-enhanced MRI, and gadoxetic acid-enhanced MRI. J Magn Reson Imaging 2011;34:326-335. [PubMed]
- Seo HJ, Kim MJ, Lee JD, Chung WS, Kim YE. Gadoxetate disodium-enhanced magnetic resonance imaging versus contrast-enhanced 18F-fluorodeoxyglucose positron emission tomography/computed tomography for the detection of colorectal liver metastases. Invest Radiol 2011;46:548-555. [PubMed]
- Donati OF, Reiner CS, Hany TF, et al. 18F-FDG-PET and MRI in patients with malignancies of the liver and pancreas. Accuracy of retrospective multimodality image registration by using the CT-component of PET/CT. Nuklearmedizin 2010;49:106-114. [PubMed]
- Oellerich M, Burdelski M, Ringe B, et al. Lignocaine metabolite formation as a measure of pre-transplant liver function. Lancet 1989;1:640-642. [PubMed]
- Merkel C, Gatta A, Zoli M, et al. Prognostic value of galactose elimination capacity, aminopyrine breath test, and ICG clearance in patients with cirrhosis. Comparison with the Pugh score. Dig Dis Sci 1991;36:1197-1203. [PubMed]
- Heymsfield SB, Fulenwider T, Nordlinger B, Barlow R, Sones P, Kutner M. Accurate measurement of liver, kidney, and spleen volume and mass by computerized axial tomography. Ann Intern Med 1979;90:185-187. [PubMed]
- van der Vorst JR, van Dam RM, van Stiphout RS, et al. Virtual liver resection and volumetric analysis of the future liver remnant using open source image processing software. World J Surg 2010;34:2426-2433. [PubMed]
- Seyama Y, Kokudo N. Assessment of liver function for safe hepatic resection. Hepatol Res 2009;39:107-116. [PubMed]
- Dello SA, van Dam RM, Slangen JJ, et al. Liver volumetry plug and play: do it yourself with ImageJ. World J Surg 2007;31:2215-2221. [PubMed]


