Association between nonalcoholic fatty liver disease and colorectal adenoma: a systemic review and meta-analysis
Introduction
Colorectal cancer (CRC) is the third most common cancer and the third leading cause of cancer death in the United States (1). For the past two decades, the incidence and mortality rate of CRC has been reduced due to the early detection and removal of precancerous polyps/adenomas by screening colonoscopy (2-4). However, the risk stratification of the target screening population needs to be further addressed in order to improve the CRC screening strategies (5,6).
In many epidemiological studies, metabolic syndrome (MetS) and its individual components, including obesity, impaired glucose tolerance, dyslipidemia, and hypertension, have been associated with an increased risk of colorectal adenoma and/or cancer (7-13). Nonalcoholic fatty liver disease (NAFLD) is considered to be a hepatic manifestation of MetS and the most common chronic liver disease worldwide (14,15). The independent relationship between NAFLD and colorectal adenoma has been investigated in multiples studies but the results have been conflicting. Some studies indicated that NAFLD was associated with a high prevalence of colorectal adenoma and/or cancer, whereas one study from USA showed no significant association (16-22). Hence we performed a systematic review and meta-analysis to evaluate the association of NAFLD with colorectal adenoma in asymptomatic patients who underwent screening colonoscopy.
Methods
Literature search and inclusion criteria
We searched the literatures of all languages from PubMed, EMBASE and the Cochrane library from January 1, 1980 through July 15, 2014, using the search terms (“nonalcoholic fatty liver disease” or “NAFLD” or “nonalcoholic steatohepatitis” or “NASH” or “fatty liver”) AND (“colorectal neoplasm” or “colorectal cancer” or “colon cancer” or “colorectal adenoma” or “colonic adenoma” or “adenomatous polyps”). The publications that meet the following criteria were included for meta-analysis: (I) they were case-control or cohort studies; (II) the study subjects were over age 18 years; (III) they reported estimates of the unadjusted and/or multivariable adjusted odds ratio (OR)/risk ratio (RR) with corresponding 95% confidence interval (CI); (IV) NAFLD patients were diagnosed at least by abdominal ultrasound or computed tomography (CT); (V) the participants were asymptomatic and undergoing screening colonoscopy.
Data extraction and quality assessment
The data was extracted independently by two reviewers and included first author, year of publication, country of study population, type of study design, sample size, number of exposed and unexposed, assessment of NAFLD, outcome measures, controlled confounders, unadjusted and/or adjusted effects estimates. We searched the reference lists of retrieved articles for additional studies. Two reviewers assessed the quality of the included studies independently by using the Newcastle-Ottawa Scale (NOS) for cohort and cross-sectional studies. The NOS judge the quality by assigning stars, with maximum of 9, based on selection of study groups, comparability of study groups, and ascertainment of either the exposure or outcome of interest. Any discrepancies regarding inclusion/exclusion and quality assessment were addressed by consensus.
Statistical analysis
For dichotomous data results from individual studies were summarized as OR and 95% CI and pooled under a random effects model. Mantel-Haenszel chi-square tests were carried out to assess the significant level of difference. Statistical heterogeneity was measured using the chi-square test and the inconsistency index (I2) statistics. The P value <0.05 or I2 >50% indicated substantial heterogeneity. The random effects model was selected if there was obvious heterogeneity; otherwise, the fixed effects model was used. The potential publication bias was assessed by visual inspection of funnel. The P value <0.05 was considered statistically significant. All analyses were performed using Review Manager 5.2. The work has been conducted and reported according to MOOSE guidelines (23).
Results
Characteristics of the studies
We initially identified a total of 403 relevant publications. After excluding 396 articles by title and abstract review, seven studies were included for further full-text review. Two studies were further excluded as they did not meet the specific inclusion criteria. Finally, five studies were included in our meta-analysis (Figure 1) (16-20).

Table 1 showed the main characteristics of the included studies. Among them, four were cross-sectional and one was retrospective cohort. Three studies were conducted in Asia, one in Europe and one in North America, with a total of 6,263 participants. All five studies had medium to high quality according to the NOS score.
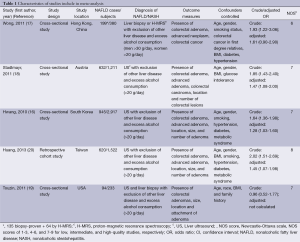
Full table
Outcome results
There was no significant heterogeneity across the five studies (P=0.24, I2 =27%), the fixed-effect model was considered to estimate the pooled OR (1.74, 95% CI: 1.53-1.97) for association between NAFLD and colorectal adenoma (Figure 2). Subgroup analyses stratified by study designs, study locations, characteristics of adenoma (location, size, number, and advanced adenoma), were also performed (Table 2). The relationship between NAFLD and colorectal adenoma was more significant in the Asian population (pooled OR =1.77, 95% CI: 1.52-2.05, n=3 studies), compared to the European/North American population (pooled OR =1.42, 95% CI: 0.75-2.67, n=2 studies). NAFLD was significantly associated with the number of colorectal adenoma (pooled OR =1.78, 95% CI: 1.10-2.86, n=2 studies), but not the location, size, or presence of advanced adenoma. Table 3 showed different comparisons regarding the type of neoplasm (adenoma or cancer) or type of exposure (NAFLD or NASH). The association between NASH and colorectal adenoma was statistically significant (pooled OR =2.54, 95% CI: 1.07-6.03, n=2 studies). Visual inspection of funnel plot did not show major asymmetrical appearance.
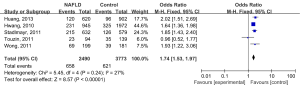
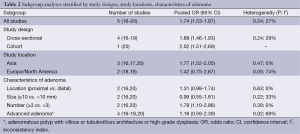
Full table

Full table
Discussion
Previous studies have shown that NAFLD is independently associated with the presence of colorectal adenoma and cancer (16-21,24). To our best knowledge, this is the first meta-analysis to investigate this relationship in asymptomatic patients undergoing screening colonoscopy.
The exact mechanisms linking NAFLD and colorectal adenoma remain unclear. NAFLD is regarded as a condition of profound insulin resistance and systemic low-grade inflammatory state (25-27). Insulin and Insulin growth factor-1 (IGF-1) have been shown to increase the risk of CRC by inhibiting apoptosis and promoting proliferation (27,28). Multiple studies have reported that tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and other pro-inflammatory cytokines play a crucial role in development of colorectal adenoma and cancer (29-31). Decreased adiponectin level and increased leptin level in NAFLD patients are also suggested to be associated with an increased risk of colorectal adenoma (25,32-34).
In our meta-analysis, we found NAFLD was significantly associated with colorectal adenoma in asymptomatic patients undergoing screening colonoscopy. However, this association was less prominent in the European/North American populations, compared to the Asian population. It may be due to the small sample size of the two studies conducted in Europe and North America. Prospective studies with larger sample size of western populations to further investigate the ethnic disparity are needed in the future. Our subgroup analyses indicated NALFD was related to more colorectal adenomas detection in screening colonoscopy. We found no association between NAFLD and CRC. Because the CRC yield rate was small in the asymptomatic patients who underwent screening colonoscopy, there was a small statistical power to detect such association. NASH which was confirmed by liver biopsy was also found to be significantly associated with colorectal adenoma.
This meta-analysis has several limitations. Firstly, the diagnosis of NAFLD in most of our studies was based on ultrasonography but not confirmed by liver biopsy. Secondly, other potential confounders, such as diet, physical activity, genetic or socioeconomic factors, etc., were not considered in the analysis. Thirdly, the majority of the participants were from Asian countries which made it difficult to assess the ethnic disparity. Lastly, only five studies were included in this meta-analysis, which made it difficult to assess the potential publication bias.
Despite these limitations, our meta-analysis has several important strengths. Firstly, we included relatively homogeneous populations who were asymptomatic and undergoing screening colonoscopy. Secondly, the definitions of exposure and outcome were also homogeneous. Lastly, the heterogeneity between studies was not significant, which increased the statistical power compared to a single study.
In conclusion, our results suggest NAFLD is significantly associated with the presence of colorectal adenoma in asymptomatic patients undergoing screening colonoscopy. More studies of western populations are needed to further investigate the ethnic disparity. Given the rising prevalence of NAFLD, we should take it into consideration when applying CRC screening strategies.
Acknowledgements
Authors’ contributions: Huafeng Shen: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript; Seth Lipka: acquisition of data, analysis and interpretation of data; Ambuj Kumar: statistical analysis, critical revision of the manuscript for important intellectual content, study supervision; Paul Mustacchia: critical revision of the manuscript for important intellectual content, study supervision.
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104-17. [PubMed]
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993;329:1977-81. [PubMed]
- Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366:687-96. [PubMed]
- Surveillance Epidemiology and End Results. 2013. Available online: http://seer.cancer.gov/statfacts/html/colorect.html#risk
- Smith RA, Brooks D, Cokkinides V, et al. Cancer screening in the United States, 2013: a review of current American Cancer Society guidelines, current issues in cancer screening, and new guidance on cervical cancer screening and lung cancer screening. CA Cancer J Clin 2013;63:88-105. [PubMed]
- Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666-89. [PubMed]
- Ahmed RL, Schmitz KH, Anderson KE, et al. The metabolic syndrome and risk of incident colorectal cancer. Cancer 2006;107:28-36. [PubMed]
- Murphy TK, Calle EE, Rodriguez C, et al. Body mass index and colon cancer mortality in a large prospective study. Am J Epidemiol 2000;152:847-54. [PubMed]
- Schiel R, Müller UA, Braun A, et al. Risk of malignancies in patients with insulin-treated diabetes mellitus: results of a population-based trial with 10-year follow-up (JEVIN). Eur J Med Res 2005;10:339-44. [PubMed]
- Lee GE, Park HS, Yun KE, et al. Association between BMI and metabolic syndrome and adenomatous colonic polyps in Korean men. Obesity (Silver Spring) 2008;16:1434-9. [PubMed]
- Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr 2007;86:s836-42. [PubMed]
- Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr 2007;86:556-65. [PubMed]
- Jinjuvadia R, Lohia P, Jinjuvadia C, et al. The association between metabolic syndrome and colorectal neoplasm: systemic review and meta-analysis. J Clin Gastroenterol 2013;47:33-44. [PubMed]
- Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 2001;50:1844-50. [PubMed]
- Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124-31. [PubMed]
- Hwang ST, Cho YK, Park JH, et al. Relationship of non-alcoholic fatty liver disease to colorectal adenomatous polyps. J Gastroenterol Hepatol 2010;25:562-7. [PubMed]
- Wong VW, Wong GL, Tsang SW, et al. High prevalence of colorectal neoplasm in patients with non-alcoholic steatohepatitis. Gut 2011;60:829-36. [PubMed]
- Stadlmayr A, Aigner E, Steger B, et al. Nonalcoholic fatty liver disease: an independent risk factor for colorectal neoplasia. J Intern Med 2011;270:41-9. [PubMed]
- Touzin NT, Bush KN, Williams CD, et al. Prevalence of colonic adenomas in patients with nonalcoholic fatty liver disease. Therap Adv Gastroenterol 2011;4:169-76. [PubMed]
- Huang KW, Leu HB, Wang YJ, et al. Patients with nonalcoholic fatty liver disease have higher risk of colorectal adenoma after negative baseline colonoscopy. Colorectal Dis 2013;15:830-5. [PubMed]
- Lee YI, Lim YS, Park HS. Colorectal neoplasms in relation to non-alcoholic fatty liver disease in Korean women: a retrospective cohort study. J Gastroenterol Hepatol 2012;27:91-5. [PubMed]
- Lin XF, Shi KQ, You J, et al. Increased risk of colorectal malignant neoplasm in patients with nonalcoholic fatty liver disease: a large study. Mol Biol Rep 2014;41:2989-97. [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [PubMed]
- Muhidin SO, Magan AA, Osman KA, et al. The relationship between nonalcoholic fatty liver disease and colorectal cancer: the future challenges and outcomes of the metabolic syndrome. J Obes 2012;2012:637538.
- Wong VW, Hui AY, Tsang SW, et al. Metabolic and adipokine profile of Chinese patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2006;4:1154-61. [PubMed]
- Targher G, Bertolini L, Scala L, et al. Non-alcoholic hepatic steatosis and its relation to increased plasma biomarkers of inflammation and endothelial dysfunction in non-diabetic men. Role of visceral adipose tissue. Diabet Med 2005;22:1354-8. [PubMed]
- Jarrar MH, Baranova A, Collantes R, et al. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2008;27:412-21. [PubMed]
- Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr 2007;86:s836-42. [PubMed]
- Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr 2001;131:3109S-20S. [PubMed]
- Gunter MJ, Canzian F, Landi S, et al. Inflammation-related gene polymorphisms and colorectal adenoma. Cancer Epidemiol Biomarkers Prev 2006;15:1126-31. [PubMed]
- Kim S, Keku TO, Martin C, et al. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res 2008;68:323-8. [PubMed]
- Hui JM, Hodge A, Farrell GC, et al. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 2004;40:46-54. [PubMed]
- Procaccini C, Galgani M, De Rosa V, et al. Leptin: the prototypic adipocytokine and its role in NAFLD. Curr Pharm Des 2010;16:1902-12. [PubMed]
- Yamaji T, Iwasaki M, Sasazuki S, et al. Interaction between adiponectin and leptin influences the risk of colorectal adenoma. Cancer Res 2010;70:5430-7. [PubMed]


