Therapeutic approaches in the management of locally advanced rectal cancer
Combined-modality therapy
Surgical resection has been the mainstay of definitive therapy for rectal cancer. Historically, recurrence rates with surgery alone were upwards of 50% (1-3). Adjuvant therapy in the form of combined post-operative radiotherapy and 5-fluorouracil (5-FU)—based chemotherapy was shown to improve local control and provide an overall survival benefit over surgery alone or surgery plus irradiation (4,5). As such, postoperative chemoradiotherapy (CRT) was recommended as the standard of care in patients with stage II (T3-T4) or stage III (node positive) rectal cancer by a National Institute of Health consensus conference in 1990 (6).
Total mesorectal excision (TME)
In addition to the incorporation of CRT, the now-widespread use of TME as pioneered by Heald et al. (7) significantly improved local recurrence (LR) rates when compared to rates using standard surgical technique. LR rates at 5 years in surgery-only arms of large randomized trials that did not mandate TME use were typically in excess of 25% (8,9), compared to 11% for surgery-only arms in trials that mandated TME use (10). When radiotherapy was added to surgical resection with standard technique, local control was improved by over 50% (local relapse rate of 11% with RT, 27% with surgery alone), and it also improved overall survival (9). Once TME was incorporated, radiotherapy had the same relative improvement in local relapse rates, but with less absolute benefit (5% with RT, 11% with TME alone) (11). Radiotherapy, when combined with TME, had a lesser absolute local control benefit, and thus failed to further increase overall survival.
Neoadjuvant chemoradiation
The current standard of care in the United States for stage II and stage III rectal cancer is neoadjuvant chemoradiation followed by surgical resection using a TME technique. The paradigm shift from postoperative to neoadjuvant therapy was largely a result of the German Rectal Cancer Study. The study randomized 823 patients with clinical stage T3-4 or node positive rectal cancer to surgery with TME followed by postoperative CRT or preoperative CRT followed by TME 6 weeks later. The preoperative regimen consisted of 50.4 Gy delivered using either a 3- or 4-field box technique with continuous-infusion 5-FU (1,000 mg/m2) on days 1-5 of weeks 1 and 5. The postoperative regimen was identical, except for a 5.4 Gy boost (55.8 Gy total) to the postoperative tumor bed. In both arms, an additional 4 cycles of bolus 5-FU (500 mg/m2 every 4 weeks) was given, starting either 4 weeks after surgery (in the preoperative group), or 4 weeks after chemoradiation (in the postoperative group).
At 5 years, there was a statistically significant lower number of LRs in the preoperative CRT arm (6% vs. 13%, P=0.006). However, there were no significant differences in the rates of distant metastases, disease-free survival, or overall survival. After preoperative CRT, there was evidence of tumor downstaging, with 8% of patients demonstrating histopathological complete response (pCR). Twenty five percent of patients receiving preoperative CRT had positive lymph nodes (compared to 40% who had surgery first in the postoperative CRT arm). Prior to randomization, every patient was evaluated by a surgeon for the need to perform an abdominoperineal resection (APR), resulting in permanent colostomy. In the group of patients deemed to require APR, preoperative CRT resulted in a higher rate of sphincter-preserving surgeries (39% vs. 19%, P=0.004) actually performed. There were fewer grade 3 or 4 acute (27% vs. 40%, P=0.001) and late toxicities (14% vs. 24%, P=0.001) in the preoperative CRT group (12). After 11 years of follow up, the significant LR benefit persisted (10-year cumulative incidence of 7.1% vs. 10.1%, P=0.048). There were also no significant differences in the 10-year cumulative incidence of distant metastases, disease-free survival and overall survival (13).
The findings of the German rectal trial were further supported by that of the National Surgical Adjuvant Breast and Bowel Project (NSABP) R-03 trial, which also compared preoperative and postoperative CRT. The radiation (45 Gy plus a 5.4 Gy boost) and chemotherapy (5-FU plus leucovorin) were identical in both arms. Surgery (TME was not mandated) followed CRT after 8 weeks in the preoperative group. The trial closed early secondary to poor accrual. Despite enrolling only 267 of a planned 900 patients, the trial demonstrated a 5-year disease-free survival improvement (64.7% vs. 53.4%) favoring preoperatively-treated patients. A pCR was achieved in 15% of the preoperative patients (14).
Shortly after publication of the landmark German study, the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for locally advanced resectable rectal cancer included neoadjuvant RT with con-current 5-FU infusion, followed by TME and an adjuvant course of consolidative chemotherapy. This approach has been widely adopted across the United States (15).
Neoadjuvant short-course radiotherapy
In some European countries, instead of preoperative CRT, a short-course of preoperative radiotherapy alone (SC-RT) is used. The Swedish Rectal Cancer Trial randomized 1,168 patients to receive 25 Gy in 5 fractions followed by surgery within 1 week, or surgery alone. TME was not mandated in this trial. At 5 years, radiotherapy reduced LRs (11% vs. 27%, P<0.001), and improved overall survival (58% vs. 48%, P<0.004) compared to surgery alone (9). After 13 years, these benefits persisted (8). The Dutch TME trial randomized 1,805 patients to be treated with or without SC-RT followed by TME. At 5 years, a LR benefit was seen (5.6% vs. 10.9%, P<0.001); however no improvement in overall survival was demonstrated. Additionally, the LR benefit was limited to those patients with negative circumferential resection margins (CRM) (10). After 12 years of follow up, the effect of SC-RT on LR persisted. In an unplanned subgroup analysis, in patients with a negative CRM, SC-RT was found to improve cancer-specific survival (50% vs. 40%, P=0.03) (11).
The Medical Research Council (MRC) in the United Kingdom and the National Cancer Institute of Canada (NCIC) randomized 1,350 patients in four countries to preoperative radiotherapy (25 Gy) or to surgery with selective postoperative CRT (45 Gy in 25 fractions with concurrent infusion 5-FU). CRT was given only to patients with positive CRM (57 of 606 patients). TME was not mandated but was performed in 92% of patients. With a median follow up of 4 years, LR was 4.4% in the preoperative SC-RT group, versus 10.6% in the selective postoperative CRT group (P<0.0001). Also noted was an improvement in disease-free survival (77.5% vs. 71.5%, P=0.013) without an overall survival benefit (16).
Neoadjuvant short-course radiotherapy versus long-course CRT
Both approaches to neoadjuvant therapy described above have shown benefits over no additional therapy and adjuvant chemoradiation. However, due to differences in eligibility criteria, efficacy comparisons between trials using different approaches are problematic. Trials that used SC-RT enrolled patients with ‘resectable’ rectal cancer (cT1-3Nx), where the CRT trials allowed only Stage II (T3-4) or Stage III (node positive) disease.
Bujko et al. were the first to conduct a randomized trial between the two neoadjuvant therapies. A total of 316 patients with clinically staged T3 or T4 rectal cancers were randomized between neoadjuvant short-course radiotherapy (25 Gy in 5 fractions) followed by TME within 7 days or “long-course” CRT (LC-CRT, 50.4 Gy in 28 fractions with concurrent 5-FU and leucovorin) with TME to follow at 4-6 weeks. Postoperative chemotherapy was allowed as indicated. This trial was powered to show a difference of 15% or greater in sphincter preservation (17,18).
After 4 years of follow up, the authors reported no significant difference in sphincter-sparing, LR (9% vs. 14% in short course and long course, respectively), or survival. Acute toxicity was higher in the CRT group (18%, compared to 3% in the radiotherapy-alone group, P<0.001). However, there was no difference in late toxicity or severe late toxicities (17).
More recently, Ngan et al. reported the outcomes of the Trans-Tasmanian Radiation Oncology Group (TROG) trial 01.04. A total of 326 patients with ultrasound or MRI-staged T3N0-2 rectal cancers were randomized between short-course preoperative radiotherapy (25 Gy in 5 fractions) followed by surgery within 1 week or long-course preoperative CRT (50.4 Gy in 28 fractions with concurrent 5-FU) followed by surgery within 4-6 weeks. Both groups received adjuvant chemotherapy (six cycles for the short-course group, four cycles for the long-course group). The trial was powered to show a 10% absolute difference in LR (15% short course, 5% long course).
After 3 years of follow up, they reported no significant difference in local relapse (7.5% for short-course, compared to 4.4% for long course, P=0.24). Additionally, no difference was seen in 5-year distant recurrences, relapse-free survival, or overall survival. There was no difference noted in sphincter-sparing. Grade 3 or 4 late toxicity, as reported at 3 years, was not different between the two groups (19).
A third randomized trial of neoadjuvant regimens is the Stockholm III trial, and is only published as an interim analysis. This study randomized 303 patients amongst 3 treatment arms. Two treatment arms used short course RT (25 Gy in 5 fractions) followed by either immediate surgery within 1 week (n=118), or delayed surgery in 4-8 weeks (n=120). Patients in the third treatment arm received long course radiotherapy (50 Gy in 25 fractions) alone, followed by surgery in 4-8 weeks. The significant finding reported in the interim analysis was the rate of postoperative complications in patients randomized to short-course radiotherapy and surgery within a week. Postoperative complications differed according to the timing of surgery relative to the start date of radiotherapy. Significantly more complications were seen in 24 of 37 (65%) patients who underwent surgery 11-17 days after the start of RT, than in 29 of 75 (39%) patients who underwent surgery less than 11 days after the start of RT (P=0.04) (20).
Without any data to-date to suggest significant differences in survival, local control, or sphincter-sparing between neoadjuvant approaches, careful study of the long-term consequence of these treatments is paramount. Quality of life (QoL) data from the Polish study is reported at 1 year after surgery, with patient-reported QoL quantified using the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 questionnaire. Anorectal and sexual function were reported using a separate questionnaire. At a median time from surgery of approximately 1 year, there were no significant differences in global function in symptoms scales for QoL between patients who received SC-RT or LC-CRT prior to surgery. There were also no differences between patient groups in answers to questions regarding anorectal or sexual function (21).
QoL data from TROG 01.04 is reported in abstract form only. Unlike that from the Polish study, 5-year data is reported. The QLQ-C30 questionnaire was used to assess global health status, and the EORTC QLQ-CR38 module was used to measure pelvic function. At 5 years, global health status was not statistically different between arms. There was no clear difference in pelvic functioning or symptoms between the SC-RT and LC-CRT arms. This data has not yet been peer-reviewed (22). Finally, a German cross-sectional study was performed in 225 patients who either underwent SC-RT (29 Gy in 10 fractions) or LC-CRT prior to surgery and were still disease-free. With a median follow-up time of 67 months, QoL analysis was performed using the EORTC QLQ-C30 and QLQ-CR29 questionnaires. Despite a modified SC-RT fractionation, there was no difference in QoL observed between patients who received SC-RT and LC-CRT, except for improved physical functioning in the LC-CRT group (23).
The debate between SC-RT and LC-CRT as the optimal preoperative regimen prior to TME is ongoing. None of the data above shows significant differences either in long-term oncologic outcomes or patient-reported QoL.
Concurrent chemotherapy with preoperative radiotherapy
Fluorouracil-based chemotherapy has long been part of adjuvant therapy for rectal cancer. The route of administration (as a continuous or bolus infusion) has been examined in several studies when CRT was given in the adjuvant setting. One intergroup study compared continuous infusion (CI) 5-FU (225 mg/m2 daily) and bolus 5-FU (500 mg/m2 daily on days 1-3 and 36-39) during adjuvant radiotherapy. CI 5-FU was associated with reduced distant metastases and improved overall survival (24). In contrast, intergroup study INT-0144 showed that CI 5-FU and bolus 5-FU during adjuvant radiation for rectal cancer resulted in no difference in three-year disease-free survival or overall survival (25).
Capecitabine is an orally-administered prodrug that is enzymatically converted to 5-FU, and was designed to mimic CI 5-FU. In a German phase III trial, 392 patients with stage II/III rectal cancer were randomized to receive either CI 5-FU or capecitabine concurrently with radiotherapy (50.4 Gy) either in the adjuvant (213 patients) or neoadjuvant (161 patients) setting. There was no difference in local relapse or overall survival. However, patients receiving capecitabine had increased rates of tumor downstaging (55% vs. 39%) and pathological node-negative rates (71% vs. 56%) compared to those receiving CI 5-FU. Patients receiving capecitabine also had significantly more hand-foot skin reactions (31% vs. 2%), but less neutropenia (35% vs. 25%) overall (26). Results of NSABP R-04 have been reported twice in abstract form so far (27,28). In this phase III trial, patients were randomized between CI 5-FU and oral capecitabine, with or without the addition of oxaliplatin (4 arm study). In both abstract reports, there were no statistical differences between pCR rate, sphincter-preservation, or surgical-downstaging. Taken together, the results of these two trials support oral capecitabine as a substitute for CI 5-FU when given concurrently with preoperative radiotherapy for rectal cancer.
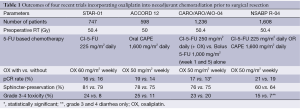
Oxaliplatin, in combination with 5-FU and leucovorin (folinic acid), as part of the FOLFOX chemotherapy regimen, plays an important role in the treatment of colorectal cancer (29). As such, several trials have investigated the addition of oxaliplatin to preoperative 5-FU-based chemoradiation. The results of these trials are shown in Table 1. In summary, the addition of oxaliplatin to concurrent preoperative CRT has shown no improvement in tumor response (based on pCR rates), or surgical outcomes (based on sphincter-preservation rates). Its addition does significantly increase the toxicity during preoperative treatment. Thus, its addition cannot be justified based on these results.
Full table
Adjuvant chemotherapy
Following neoadjuvant CRT and surgical resection for stage II/III rectal cancer, the NCCN Guidelines recommend adjuvant chemotherapy regardless of the surgical pathology results. Despite limited data demonstrating the efficacy of this approach, adherence to this recommendation is fairly high. A recent study of adjuvant chemotherapy use at several NCCN institutions between 2005 and 2010 showed that of 1,193 patients who received neoadjuvant therapy, 990 (83%) were also prescribed and initiated further adjuvant chemotherapy (30). Of the remaining patients, the most frequent reason for not recommending chemotherapy was comorbid illness (25 of 50 patients). The most frequent reason that chemotherapy was recommended but not received by the patient was patient refusal (54 of 74 patients).
Most of the evidence for the role adjuvant chemotherapy is from older studies using postoperative therapy alone. EORTC trial 22921 was a four-armed study comparing preoperative radiotherapy (45 Gy in 25 fractions) with or without concurrent chemotherapy (5-FU and leucovorin) and adjuvant chemotherapy (4 or more cycles, every 3 weeks). A total of 1,011 patients were randomized; 787 patients who had an R0 surgical resection with no distant spread before or at surgery were eligible for analysis of outcome by adjuvant treatment. In the initial report, there was no effect of adjuvant chemotherapy on disease-free survival or overall survival for the group as a whole. Adherence to postoperative chemotherapy was poor (43% of patients received at least 95% of the planned fluorouracil without delay) (31). Later, an unplanned subgroup analysis was published, showing a statistically significant survival benefit in patients who underwent tumor downstaging (ypT0-2) from neoadjuvant therapy (32). Long-term results (median follow up of 10.4 years) showed no difference in disease-free survival or overall survival in patients with tumor pathological downstaging, those without, or the group as a whole (33).
Adjuvant chemotherapy, for now, remains as part of recommended therapy in the United States. At several NCCN institutions, the rate of adjuvant chemotherapy prescription and initiation is quite high. However, with increased toxicity, poor adherence to the full prescription course and limited evidence of its benefit, newer clinical trials appear to be shifting further chemotherapy upfront instead of the adjuvant setting.
Neoadjuvant chemotherapy and CRT (or radiotherapy)
The EORTC study above and others (34) have concluded that the addition of chemotherapy to ‘long-course’ preoperative radiotherapy significantly improved local control. Local control has improved to the point that distant relapses are the more common site of first recurrence. With poor adherence to adjuvant chemotherapy and little evidence of its value, the role of neoadjuvant chemotherapy prior to neoadjuvant CRT is being actively investigated. Potential advantages of upfront chemotherapy include improved compliance, and the early treatment of micrometastases.
One phase II trial (Expert) out of the United Kingdom enrolled patients with high risk disease (based on CRM margin risk, low-lying tumors, T4 and/or node positive tumors) to receive 12 weeks of neoadjuvant capecitabine and oxaliplatin (CAPOX) followed by single-agent (capecitabine) CRT (54 Gy), TME, and four cycles of postoperative adjuvant capecitabine. A total of 105 eligible patients were enrolled. A total of 95 patients underwent TME, of whom 21 had a pCR (20% of eligible patients). Three-year progression-free and overall survival were 68% (95% CI, 59-77) and 83% (95% CI, 76-91), respectively. The authors report acceptable safety despite nine cardiac or thromboembolic events (9%) of which four died, requiring amendment of the protocol for cardiovascular safety (35).
Another randomized, phase II Spanish trial (Grupo Cancer de Recto 3 Study) randomized 108 patients with locally advanced rectal cancer to receive either preoperative CRT (50.4 Gy with concurrent capecitabine and oxaliplatin) followed by TME and postoperative chemotherapy (capecitabine-oxaliplatin), or ‘induction’ chemotherapy (capecitabine-oxaliplatin) followed by the same CRT and TME (no postoperative chemotherapy). The group of patients that received induction chemotherapy had greater chemotherapy dose exposure than those patients that received adjuvant chemotherapy. However, there was no statistical difference between pCR rate (13.5% and 14.3%), downstaging, tumor regression, or R0 resection. Grade 3 and 4 toxicities were similar in both arms during CRT. Toxicity was compared between the adjuvant chemotherapy window in the first group and the induction chemotherapy window in the second group. Despite a greater chemotherapy exposure for patients who received induction chemotherapy, there was greater grade 3 and 4 toxicity during adjuvant chemotherapy (54% vs. 37%, respectively, P=0.0004) (36).
Another approach being investigated in phase III studies, is the use of short-course radiotherapy (25 Gy in 5 fractions), followed by neoadjuvant capecitabine-oxaliplatin chemotherapy and TME. This approach is the experimental arm in both the Rectal Cancer and Preoperative Induction Therapy Followed by Dedicated Operation (RAPIDO) trial, and a Polish Colorectal Cancer Study Group (5-FU, leucovorin and oxaliplatin chemotherapy) trial. The standard arm in these trials is long-course CRT. It will be imperative for both trials to carefully detail not only differences in outcomes, but also toxicity (acute, late and post-surgical complications) and QoL to definitively differentiate the two approaches.
Neoadjuvant chemotherapy alone
In the TME era, with high-quality MRI and ultrasound staging, the option for omitting preoperative radiotherapy in carefully selected patients has been raised. Preliminary, pilot data out of Memorial Sloan-Kettering Cancer Center treated 32 patients with FOLFOX (5-FU, leucovorin and oxaliplatin) plus bevacizumab alone followed by TME. Pathologic complete response rate was 25% with a 4-year LR rate and disease-free survival of 0% and 84%, respectively (37).
These exciting results have prompted the preoperative radiation or selective preoperative radiation and evaluation before chemotherapy and TME (PROSPECT or N1048) trial. In this multi-institution, phase II/III study, only patients with ‘low-risk’ Stage II/III rectal cancer [candidates for sphincter-sparing surgeries, CRM not-threatened, non-T4 tumors, clinically node-positive disease must be N1 (1-3 nodes) only] are eligible. Patients are randomized to one of two treatment arms. Group 1 patients receive six cycles of FOLFOX alone followed by restaging. Patients with a greater than 20% tumor regression proceed to surgery with TME. Patients with a less than 20% tumor response undergo CRT followed by TME. Group 2 receives standard-of-care neoadjuvant CRT, followed by TME. Patients in both groups may receive adjuvant chemotherapy.
Targeted therapies
Bevacizumab, a humanized monoclonal antibody against vascular endothelial growth factor, has been studied in phase I and II trials incorporating it with conventional preoperative 5-FU based CRT. The data so far has shown encouraging pCR rates (16-32%) (38-41), but several studies report increased rates of postoperative wound complications (38-42).
Cetuximab and panitumumab are both humanized monoclonal antibodies against epidermal growth factor receptor approved for use in patients with metastatic colorectal cancer. Phase I/II trials with cetuximab use in preoperative CRT for rectal cancer, as a whole, have shown mixed efficacy with not-insignificant grade 3-4 gastrointestinal toxicity (43). One randomized phase II clinical trial (EXPERT-C) was conducted following a previous trial (EXPERT) looking at neoadjuvant chemotherapy, followed by chemoradiation then surgery. In the EXPERT-C trial, 165 patients received capecitabine-oxaliplatin chemotherapy, followed by capecitabine CRT with or without cetuximab, then TME. In tumors with wild-type k-ras, addition of cetuximab did not improve the primary endpoint of pCR or progression-free survival. Cetuximab did improve response rates and 3-year overall survival (HR 0.27, P=0.034) (44).
The effect of these targeted therapies on long-term outcomes and side-effects requires further study, although the mixed results thus far have been disappointing.
IMRT for rectal cancer
As seen in all of the studies described here, the ability of patients to adhere to treatment schedules and complete full courses of chemotherapy and CRT is a major issue. The most common radiation-induced toxicities are skin and gastrointestinal (diarrhea)-related. Intensity-modulated radiotherapy (IMRT) use in other disease sites within the pelvis, such as prostate, anus and GYN, has been shown to reduce treatment-related morbidities (45-47).
Thus far, evidence for IMRT use in rectal cancer has been building. One dosimetric study has shown that IMRT, when compared to 3D-conformal radiotherapy (3D), reduces the volume of small bowel receiving 15 Gy or higher (V15) (48), a factor shown to be associated with increased rates of Grade 3 diarrhea (49). Another dosimetric study showed that the small bowel V15 is improved, even if the patient is treated in the prone position with a belly board (a device often used to displace small bowel out of the radiation field (50). Clinical data, to-date, consists mostly retrospective series showing reduction in grade 2 or higher GI toxicity and diarrhea (51,52). A recently completed phase II study, RTOG 08-22, examined the role of preoperative radiotherapy using IMRT concurrently with capecitabine and oxaliplatin, and results are pending.
Conclusions
In the treatment of locally advanced rectal cancer, major paradigm shifts such as the TME surgical technique and the use of neoadjuvant therapy instead of adjuvant, have led to significant advances in the local control and overall survival of these patients. In the United States and several European countries, the standard of care is neoadjuvant CRT followed by surgery with TME and adjuvant chemotherapy. In some countries, short-course radiotherapy, in lieu of CRT, is used. In that case, surgery follows immediately (within 1 week) as opposed to a 4-8 weeks after CRT. Two major phase III trials have compared these two approaches, neither of which found any differences in oncologic or QoL outcomes. A clear theme from several studies included in this review, is that adjuvant therapy adds to patient toxicity. The toxicity of adjuvant chemotherapy has resulted in low adherence to the protocols, and there does not appear to be a clear benefit to this approach. In the modern era of more accurate MRI and/or ultrasound staging, and newer chemotherapeutic drugs and targeted therapies, recent research has attempted to incorporate them into the neoadjuvant setting with mixed success. Current ongoing trials seek to use more aggressive chemotherapy up front, with or without radiotherapy or CRT prior to surgery. Going forward, it will be imperative to balance aggressive therapy to control local relapse and distant metastases with long-term toxicity and effects on patient QoL, as these patients live longer after surviving their disease. It is important to continue to investigate treatments to maximize therapeutic effect (neoadjuvant FOLFOX, targeted drugs), but also to minimize toxicity (IMRT use).
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Gunderson LL, Sosin H. Areas of failure found at reoperation (second or symptomatic look) following “curative surgery” for adenocarcinoma of the rectum. Clinicopathologic correlation and implications for adjuvant therapy. Cancer 1974;34:1278-92. [PubMed]
- Cass AW, Million RR, Pfaff WW. Patterns of recurrence following surgery alone for adenocarcinoma of the colon and rectum. Cancer 1976;37:2861-5. [PubMed]
- Gilbertsen VA, Nelms JM. The prevention of invasive cancer of the rectum. Cancer 1978;41:1137-9. [PubMed]
- Douglass HO Jr, Moertel CG, Mayer RJ, et al. Survival after postoperative combination treatment of rectal cancer. N Engl J Med 1986;315:1294-5. [PubMed]
- Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 1991;324:709-15. [PubMed]
- NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA 1990;264:1444-50. [PubMed]
- Heald RJ. A new approach to rectal cancer. Br J Hosp Med 1979;22:277-81. [PubMed]
- Folkesson J, Birgisson H, Pahlman L, et al. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005;23:5644-50. [PubMed]
- Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med 1997;336:980-7. [PubMed]
- Peeters KC, Marijnen CA, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg 2007;246:693-701. [PubMed]
- van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011;12:575-82. [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [PubMed]
- Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33. [PubMed]
- Roh MS, Colangelo LH, O’Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009;27:5124-30. [PubMed]
- Reyngold M, Niland J, ter Veer A, et al. Neoadjuvant radiotherapy use in locally advanced rectal cancer at NCCN member institutions. J Natl Compr Canc Netw 2014;12:235-43. [PubMed]
- Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet 2009;373:811-20. [PubMed]
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006;93:1215-23. [PubMed]
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol 2004;72:15-24. [PubMed]
- Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30:3827-33. [PubMed]
- Pettersson D, Cedermark B, Holm T, et al. Interim analysis of the Stockholm III trial of preoperative radiotherapy regimens for rectal cancer. Br J Surg 2010;97:580-7. [PubMed]
- Pietrzak L, Bujko K, Nowacki MP, et al. Quality of life, anorectal and sexual functions after preoperative radiotherapy for rectal cancer: report of a randomised trial. Radiother Oncol 2007;84:217-25. [PubMed]
- Ngan S, Fisher R, Burmeister B, et al. Long-term Quality of Life in patients treated in TROG 01.04: a randomized trial comparing short course and long course preoperative radiation therapy for rectal cancer. Int J Radiat Oncol 2012;84:s143-4.
- Guckenberger M, Saur G, Wehner D, et al. Long-term quality-of-life after neoadjuvant short-course radiotherapy and long-course radiochemotherapy for locally advanced rectal cancer. Radiother Oncol 2013;108:326-30. [PubMed]
- O’Connell MJ, Martenson JA, Wieand HS, et al. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med 1994;331:502-7. [PubMed]
- Smalley SR, Benedetti JK, Williamson SK, et al. Phase III trial of fluorouracil-based chemotherapy regimens plus radiotherapy in postoperative adjuvant rectal cancer: GI INT 0144. J Clin Oncol 2006;24:3542-7. [PubMed]
- Hofheinz RD, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 2012;13:579-88. [PubMed]
- Roh MS, Colangelo LH, O’Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009;27:5124-30. [PubMed]
- Allegra CJ, Yothers G, O’Connell MJ, et al. Neoadjuvant therapy for rectal cancer: Mature results from NSABP protocol R-04. J Clin Oncol 2014;32:abstr 390.
- Sanoff HK, Carpenter WR, Martin CF, et al. Comparative effectiveness of oxaliplatin vs non-oxaliplatin-containing adjuvant chemotherapy for stage III colon cancer. J Natl Cancer Inst 2012;104:211-27. [PubMed]
- Khrizman P, Niland JC, ter Veer A, et al. Postoperative adjuvant chemotherapy use in patients with stage II/III rectal cancer treated with neoadjuvant therapy: a national comprehensive cancer network analysis. J Clin Oncol 2013;31:30-8. [PubMed]
- Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med 2006;355:1114-23. [PubMed]
- Collette L, Bosset JF, den Dulk M, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol 2007;25:4379-86. [PubMed]
- Bosset JF, Calais G, Mineur L, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol 2014;15:184-90. [PubMed]
- Gérard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 2006;24:4620-5. [PubMed]
- Chua YJ, Barbachano Y, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol 2010;11:241-8. [PubMed]
- Fernández-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol 2010;28:859-65. [PubMed]
- Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol 2014;32:513-8. [PubMed]
- Willett CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol 2009;27:3020-6. [PubMed]
- Resch G, De Vries A, Öfner D, et al. Preoperative treatment with capecitabine, bevacizumab and radiotherapy for primary locally advanced rectal cancer--a two stage phase II clinical trial. Radiother Oncol 2012;102:10-3. [PubMed]
- Spigel DR, Bendell JC, McCleod M, et al. Phase II study of bevacizumab and chemoradiation in the preoperative or adjuvant treatment of patients with stage II/III rectal cancer. Clin Colorectal Cancer 2012;11:45-52. [PubMed]
- Crane CH, Eng C, Feig BW, et al. Phase II trial of neoadjuvant bevacizumab, capecitabine, and radiotherapy for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2010;76:824-30. [PubMed]
- Uehara K, Hiramatsu K, Maeda A, et al. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor-risk rectal cancer: N-SOG 03 Phase II trial. Jpn J Clin Oncol 2013;43:964-71. [PubMed]
- Glynne-Jones R, Mawdsley S, Harrison M. Cetuximab and chemoradiation for rectal cancer--is the water getting muddy? Acta Oncol 2010;49:278-86. [PubMed]
- Dewdney A, Cunningham D, Tabernero J, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J Clin Oncol 2012;30:1620-7. [PubMed]
- Kachnic LA, Winter K, Myerson RJ, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 2013;86:27-33. [PubMed]
- Zelefsky MJ, Fuks Z, Hunt M, et al. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys 2002;53:1111-6. [PubMed]
- Mundt AJ, Lujan AE, Rotmensch J, et al. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys 2002;52:1330-7. [PubMed]
- Robertson JM, Lockman D, Yan D, et al. The dose-volume relationship of small bowel irradiation and acute grade 3 diarrhea during chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 2008;70:413-8. [PubMed]
- Engels B, De Ridder M, Tournel K, et al. Preoperative helical tomotherapy and megavoltage computed tomography for rectal cancer: impact on the irradiated volume of small bowel. Int J Radiat Oncol Biol Phys 2009;74:1476-80. [PubMed]
- Kim JY, Kim DY, Kim TH, et al. Intensity-modulated radiotherapy with a belly board for rectal cancer. Int J Colorectal Dis 2007;22:373-9. [PubMed]
- Parekh A, Truong MT, Pashtan I, et al. Acute gastrointestinal toxicity and tumor response with preoperative intensity modulated radiation therapy for rectal cancer. Gastrointest Cancer Res 2013;6:137-43. [PubMed]
- Samuelian JM, Callister MD, Ashman JB, et al. Reduced acute bowel toxicity in patients treated with intensity-modulated radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 2012;82:1981-7. [PubMed]


