Survival trends of metastatic small intestinal neuroendocrine tumor: a population-based analysis of SEER database
Introduction
Neuroendocrine cells are dispersed throughout the body and neoplasms arising from these specialized cells are classified as neuroendocrine tumors (NETs). NETs consist of a spectrum of malignancies defined as epithelial neoplasms with principally neuroendocrine differentiation. Even though these tumors have been recognized for decades, there are many unanswered questions about the incidence, diagnosis, prognosis and treatment options. In 2010, the World Health Organization (WHO) classified these tumors and replaced “carcinoid” with the terms NET grade 1 (G1), NET grade 2 (G2) and grade 3 (G3) neuroendocrine carcinomas (NECs) based on mitotic rate and the Ki-67 index (1-3). More than 50% of NETs originate in the gastrointestinal system or pancreas (4). Amongst various locations, incidence rates of small intestinal NETs (SNETs) are increasing significantly over the last few decades. The age-adjusted incidence rate increased 6.4-fold from 1973 (1.09 per 100,000) to 2012 (6.98 per 100,000) (5), which is likely secondary to improvement in the diagnostic techniques for NETs, such as computed tomography and endoscopy (5,6). NETs are now the most frequent type of small intestinal tumors (7).
SNETs are mostly indolent; however, can be aggressive and already metastasized at the time of diagnosis. Symptoms for patients with the early stage disease are often vague and subtle. More specific symptoms would only develop at a later stage when the tumor is big enough to cause small bowel obstruction or when the imaging techniques detect the primary tumor or metastatic lesions. Unfortunately, SNETs are commonly diagnosed at stage IV, with distant organ metastasis which is most frequently found in the liver (50–75% of cases) (8). Five-year survival rates reported in various studies for metastatic SNETs ranges from 46% to 75% (9-12) depending on differentiation and various other factors.
Current National Comprehensive Cancer Network (NCCN) guidelines for metastatic SNETs suggest resection of the primary tumor and metastatic sites if complete resection is possible (13). If complete resection is not possible, the recommendation is to use nonsurgical management such as somatostatin analogues, everolimus and sunitinib, chemotherapy, peptide receptor radionuclide therapy (PRRT) or watchful waiting depending on tumor burden and symptoms. The most recent European Neuroendocrine Tumor Society (ENETS) and North American Neuroendocrine Tumor Society (NANETS) recommend resection of well differentiated tumors (G1, G2) and systemic therapy for poorly or undifferentiated tumors (G3), in the presence of metastasis. There are significant controversies and differences in the guidelines on the resection of asymptomatic NETs in the presence of unresectable liver metastases or asymptomatic primary tumors (13-16).
A recent retrospective study showed that surgical resection of the primary tumor significantly improves survival in early SNET without organ metastasis (HR 0.50, 95% CI: 0.28–0.90, P=0.01) (17). Yet, the impact of resection of the primary tumor in the patients with metastatic SNET is widely controversial. In this setting, some studies showed that the surgery for primary tumor improves survival (18,19), while others showed no difference (10). The aim of our study is to evaluate survival trends in patients with metastatic SNETs based on various patient and tumor characteristics stratified by the type of therapy patients received. We accessed and analyzed data on metastatic SNET patients from 2000–2014, from the Surveillance, Epidemiology, and End results (SEER) database (20).
Methods
The SEER database is derived from cancer registries representing approximately 28% of the U.S. population and is maintained by the National Cancer Institute (www.seer.cancer.gov) (20). The SEER population-based cancer registries contain information on cancer incidence and survival in selected geographic areas. Selection of the included geographic areas was based on the quality of their cancer reporting systems and population diversity.
A retrospective cohort study was performed using data from the SEER database, based on the November 2016 submission, which was released in April 2017. Data was examined from 2000 through 2014 from eighteen SEER registries. The SEER data set includes information on patient demographics, tumor characteristics, cancer-associated treatments, use of cancer directed surgery, and survival for individuals with cancer. Surgical intervention is coded in the SEER database as a separate variable, whenever it is performed. The actual surgical procedure directed at the primary site is coded as a separate variable. No record of chemotherapy is available in this database.
Study population
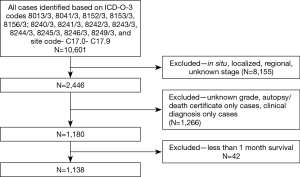
SEER*Stat version 8.3.2 was used for all data collection and survival analysis. Patient inclusion criteria based on the site recode International Classification of Diseases for Oncology, third Edition (ICD-O-3) [2008] for SNET (C 17.0–C 17.9) and year of diagnosis (2000 through 2014). SNETs are defined by the following ICD-O-3 histology codes for malignant cases: 8013/3 [large cell neuroendocrine carcinoma (NEC)], 8041/3 (small cell NEC), 8152/3 (glucagonoma), 8153/3 (gastrinoma), 8156/3 (somatostatinoma); 8240/3 (carcinoid), 8241/3 (enterochromaffin cell carcinoid), 8242/3 (enterochromaffin-like cell tumors), 8243/3 (goblet cell carcinoid), 8244/3 (mixed adenoneuroendocrine carcinoma), 8245/3 (adenocarcinoid), 8246/3 (NEC), and 8249/3 (atypical carcinoid). The extent of disease is categorized by SEER Summary staging manual [2000], as localized, regional disease, and distant disease. We aimed at analyzing patients with metastatic NETs; hence, patients with localized, regional and unknown stage were excluded. Only actively followed or treated cases were included. Cases identified on autopsy or reported only on a death certificate were excluded. Diagnosis made based on only clinical suspicion without radiological, laboratory or microscopic confirmation were excluded. Patients with death reported within the first month of diagnosis were excluded, as SEER data reports their survival as zero months. Step by step patient selection process is shown in Figure 1.
For analysis purpose, age group was divided into two: o50 and >50 years. Patient race was categorized as white, black, and other based on SEER coding schemeJeny (20). Histology was classified into carcinoid tumor (8240/3) which is NET G1 as per WHO classification, neuroendocrine cancer (8013/3, 8041/3, 8246/3) which is NEC as per WHO classification, and other types (8152/3, 8153/3, 8156/3, 8241/3, 8242/3, 8243/3, 8244/3, 8245/3, 8249/3). The grade of the tumor is categorized as well differentiated (Grade 1), moderately differentiated (Grade 2), poorly differentiated (Grade 3) and undifferentiated (Grade 4). For calculation purpose, we combined poorly and undifferentiated in a common group (Grade 3/4). Therapy received was categorized using SEER site-specific therapy of primary site codes and divided into two main groups: surgical resection and no surgery. To further characterize and analyze the impact of the type of surgery, the group which received surgery was divided into two subgroups: local resection (LR) and radical resection (RR). LR was defined as simple resection, polypectomy, and excisional biopsy, excision of lesion, simple removal of the lesion, or partial removal of the lesion. RR was defined as debulking or RR with an en bloc resection (partial or total removal) of other organs (21).
Statistical analysis
Descriptive statistics for categorical variables were analyzed using proportions, and Chi-square test of independence, whereas continuous variables were described using means and medians. Missing observations were imputed using the multiple imputation procedure available in Stata statistical software (22). Non-parametric Kaplan-Meier survival estimates and Cox proportional hazard models were used for this analysis. The log-rank test was used to estimate equality of survival curves. Relative survival rates (RSRs) are estimated as the ratio of the overall survival to expected survival. As the hazard function can be used to obtain the survival function, the overall survival is the product of the expected survival and the relative survival. These models estimate the excess hazard rate in the patients with a particular disease characteristic compared with the expected hazard rate in the general population. Stata statistical software, release 14 (22) and R Statistical Software (23) were used for analysis.
Results
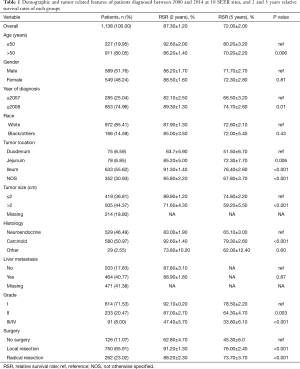
We identified a total of 1,138 SNET cases that met the inclusion criteria from SEER database. Demographic features are mentioned in Table 1.
Full table
The median age of patients was 61 years. The median relative survival of entire patient population was 41 months with 5 years RSR of 72.00%. Survival significantly improved for those diagnosed after 2008 (5 years RSR 66.50% vs. 74.70%, respectively for those diagnosed between 2000–2007 and 2008–2014, P<0.01). Survival rates were significantly lower for the age group >50 years (5 years RSR 80.20% vs. 70.20%, respectively for ≤5 and >50 years, P=0.006). Primary tumor in the duodenum and tumor size >2 cm had the worst survival with compared to other parts of small intestine and tumor size ≤2 cm (P<0.001 and P<0.001, respectively. Furthermore, poor differentiation was associated with poor outcome as shown in Table 1. Gender, race, and presence of liver metastasis at the time of diagnosis did not impact survival.
Multivariable analysis
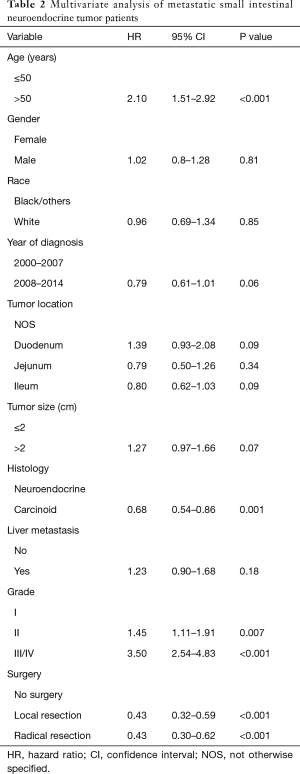
Age >50 years (HR 2.10, 95% CI: 1.51–2.92, P<0.001) and poorly differentiated histology (HR 3.50, 95% CI: 2.54–4.83, P<0.001) continued to show poor outcome even with multivariable analysis by Cox regression analysis as shown in Table 2. While there was a trend towards better outcome since 2008, it was not statistically significant on multivariable analysis (HR 0.79, 95% CI: 0.61–1.01, P=0.06). Similarly, tumor size >2 cm continued to show trend towards poor outcome (HR 1.27, 95% CI: 0.97–1.66, P=0.07). Carcinoid histology showed better outcome (HR 0.68, 95% CI: 0.54–0.86, P=0.001) compared to NEC histology.
Full table
Survival based on surgery
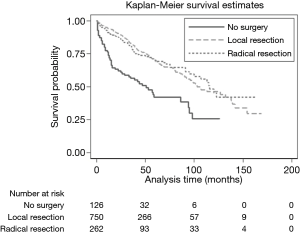
Vast majority (89%) of metastatic SNET patients received surgical resection. The group which did not receive any tumor-directed surgery showed the worst survival (5 years RSR 45.30% vs. 76%, respectively for no surgery vs. surgery group, P<0.001) (Figure 2). This trend was consistent even on multivariable analysis (HR 0.43, 95% CI: 0.32–0.59, P<0.001). Upon further stratification, surgery seems to improve outcome irrespective of various patient and tumor characteristics but benefit was most pronounced in patients age >50 years (HR 0.37), primary tumor location at duodenum (HR 0.13), tumor size ≤2 cm (HR 0.26), poorly or undifferentiated histology (HR 0.29) (Figure 3). Amongst the patients who received surgery (N=1,012), LR was performed on 750 (74.11%) patients while RR was performed on 262 (25.88%) patients. We found no significant difference in survival between LR and RR (HR 1.01, 95% CI: 0.73–1.40, P=0.92).
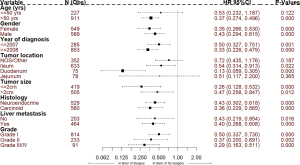
Liver metastasis
Among the patient who had liver metastases at the time of presentation (N=464), we measured the survival difference between LR and RR. We found no survival difference between LR and RR in this group of patients (5 years RSR 80.7% vs. 75.6%, P=0.61, respectively by LR and RR).
Discussion
To our knowledge, this is the first large population-based study that studies the patient characteristics and survival trends in metastatic SNETs. We found the 5-year RSR for metastatic SNET was 72% which is comparable with other published reports of metastatic SNETs (9-11,24). Our analysis also shows that the survival has significantly improved over last few years (5 years RSR 66.5% vs. 74.7%, respectively for those diagnosed before 2008 and after 2008, P<0.01). This is likely from multiple factors such as disease awareness in patients, physicians, sophisticated diagnostic tools, improvement in the surgical techniques, improved health care access, and advancement in liver-directed therapies. We found that age >50 years, duodenal location, tumor size >2 cm, poor differentiation of the tumor and not receiving any surgical intervention were found to have poor outcomes. Gender, race and liver metastasis at the time of diagnosis did not impact survival in metastatic SNETs.
Due to lack of randomized controlled trials, the guidelines and recommendations for management of SNETs are based on evidence produced by retrospective analyses or anecdotal experience. ENETS and NANETS recommend resection of primary tumors in the small intestine, if patients are symptomatic from the burden causing obstruction or concern for impending obstruction. There is no clear guideline on the impact of resection of asymptomatic NETs in the presence of unresectable liver metastases or asymptomatic primary tumors (16) and its impact on survival. Our analysis suggests significant survival benefit in patients who received surgery for the primary tumor (either LR or RR) (HR 0.43, 95% CI: 0.32–0.59, P<0.001) even in presence of metastasis. One possible explanation for this improved survival is that the patients who received surgery could have had more limited metastatic spread of disease compared to patients who did not have surgery. This is a limitation related to the retrospective nature of the study based on SEER database. These results are comparable to previously published results of survival benefit of primary tumor resection even in metastatic SNET (9,19,25). Further analysis suggested that the benefits of surgery was noted regardless of tumor size, histology, grade or liver metastasis, but the greatest benefit was seen in patients with age >50 years and primary duodenal tumor. This supports previously reported findings that surgery to remove the primary intestinal tumor improves survival even in the presence of unresectable liver metastases (26).
The impact of the type of surgery (LR vs. RR) on survival is still a subject of debate. NCCN guidelines recommend complete resection of the primary and metastases, if possible. But if complete resection is not possible, there is a wide array of options from watchful waiting or consideration of surgery or non-surgical management. Moreover, often it becomes hard to identify all the tumor sites with conventional imaging techniques before surgery as tumor multicentricity is common for SNET. The retrospective analysis done to answer this question has shown conflicting results (19,24,27). Landerholm et al. (27) reported incomplete tumor resection (HR 2.71, 95% CI: 1.11–6.61) was associated with worse disease-specific survival in patients with small bowel carcinoid tumors. However, our analysis shows no difference in survival between LR and RR (HR 1.01, 95% CI: 0.73–1.40, P=0.92).
Moreover, the impact of liver resection or liver-directed therapies on survival of SNET patients with liver metastasis is unclear with conflicting results (18,24,28,29). In one retrospective analysis, patients who underwent hepatic resection had a significant survival benefit; median survival was 11.26 years (95% CI: 5.19–17.33) for those who had resection vs. 5.5 years (95% CI: 4.33–6.17) for those who did not (18). In another analysis, patients who underwent liver resection or radiofrequency ablation for liver metastatic SNETs did not show any survival advantage compared to the non-intervention control group (24). This variation could partly be secondary to the retrospective nature of the studies leading to selection bias. In our analysis, RR does not show any survival benefit as mentioned in results. However, it is important to know that SEER does not provide the details of the type of liver surgery, the extent of liver metastasis, the extent of resection and any co-existing liver disease; as all these factors can affect the overall survival of patient after such intervention. Nonetheless, our analysis suggests the need for further studies preferably prospective trials to clarify the roles of recent advancements in liver-directed surgery such as radiofrequency ablation, arterial embolization, hepatic chemoembolization, or cytoreductive surgery in this group of patients. Several recent diagnostic tools such as 68-Gallium DOTA peptide (DOTATATE, DOTATOC and DOTANOC) positron emission tomography (PET)-CT, which has high sensitivity for detecting NETs, may help to define the resectability in SNETs (30).
Our study has a few limitations: (I) while quite detailed, SEER database lacks in information about various factors such as functional status of patients, symptom burden, Ki-67 index, extent of metastasis and other comorbidities, all of which can impact the candidacy for surgery and thus survival in this specific group of patients with metastatic SNET; (II) as mentioned above, SEER does not provide the details of any co-existing liver disease, the extent of liver metastasis and the type of liver-directed surgery; and all these factors may affect the overall survival in metastatic SNET; (III) SEER lacks the details of imaging modality of choice for staging purpose and systemic therapies provided; (IV) we categorized SEER surgery codes for surgical debulking and partial or total removal of other organs of metastasis as RR. However, SEER does not specify on which organs and partial vs. total removal.
In conclusion, surgery for the primary tumor showed a significant survival advantage in SNET patients even in the presence of distant organ metastasis. Survival seems to have improved since 2008, likely from improvement in disease awareness, sophisticated diagnostic tools, and multidisciplinary team approach involving medical, surgical, radiation and interventional radiology therapies. Age >50 years, duodenal location of the primary tumor, tumor size >2 cm, and poor differentiation of the tumor were found to be poor prognostic factors. Gender, race and liver metastasis at the time of diagnosis did not impact survival in this group of patients. No difference in survival was found between LR and RR. Moreover, the benefit of primary tumor resection was seen irrespective of primary tumor size, grade, histology or liver metastasis, while the greatest benefit was seen in >50 years patients and primary duodenal tumor. We believe that treatment of metastatic SNETs is a therapeutic challenge, which warrants a multidisciplinary approach. This analysis provides information on identifying at-risk populations to help learn the impact on survival of current therapeutic options which in turn can help clinicians in their therapeutic decisions. The data also provides direction for further research in this complex medical condition.
Acknowledgments
None.
Footnote
Conflicts of Interest: SR Chandana—Participates in Advisory Board and Speaker Bureau programs for IPSEN Biopharmaceuticals. The other authors have no conflicts of interest to declare.
Ethical Statement: No formal approval of IRB is required as data were collected from a source that was publicly available and did not contain unique patient identifiers. We obtained permission to access research data files of SEER database with the reference number 12327-Nov2016. Given that these data are de-identified and ethics approval is waived, the study did not require informed consent.
References
- Bosman FT, Carneiro F, Hruban RH, et al. WHO Classification of Tumours of the Digestive System, Fourth Edition. Lyon France: IARC Press, 2010.
- Kim JY, Hong SM. Recent updates on neuroendocrine tumors from the gastrointestinal and pancreatobiliary tracts. Arch Pathol Lab Med 2016;140:437-48. [Crossref] [PubMed]
- Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci 2004;1014:13-27. [Crossref] [PubMed]
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol 2017;3:1335-42. [Crossref] [PubMed]
- Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143:1179-1187.e3. [Crossref] [PubMed]
- Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg 2009;249:63-71. [Crossref] [PubMed]
- Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9:61-72. [Crossref] [PubMed]
- Givi B, Pommier SJ, Thompson AK, et al. Operative resection of primary carcinoid neoplasms in patients with liver metastases yields significantly better survival. Surgery 2006;140:891-7; discussion 897-8. [Crossref] [PubMed]
- Strosberg J, Gardner N, Kvols L. Survival and prognostic factor analysis of 146 metastatic neuroendocrine tumors of the mid-gut. Neuroendocrinology 2009;89:471-6. [Crossref] [PubMed]
- Capurso G, Rinzivillo M, Bettini R, et al. Systematic review of resection of primary midgut carcinoid tumour in patients with unresectable liver metastases. Br J Surg 2012;99:1480-6. [Crossref] [PubMed]
- Norlén O, Stålberg P, Öberg K, et al. Long-term results of surgery for small intestinal neuroendocrine tumors at a tertiary referral center. World J Surg 2012;36:1419-31. [Crossref] [PubMed]
- Clark OH, Benson AB 3rd, Berlin JD, et al. NCCN Clinical Practice Guidelines in Oncology: neuroendocrine tumors. J Natl Compr Canc Netw 2009;7:712-47. [Crossref] [PubMed]
- Boudreaux JP, Klimstra DS, Hassan MM, et al. (NANETS) NANTS. The NANETS consensus guideline for the diagnosis and management of neuroendocrine tumors: well-differentiated neuroendocrine tumors of the Jejunum, Ileum, Appendix, and Cecum. Pancreas 2010;39:753-66. [Crossref] [PubMed]
- Pavel M, Baudin E, Couvelard A, et al. ENETS Consensus Guidelines for the Management of Patients with Liver and Other Distant Metastases from Neuroendocrine Neoplasms of Foregut, Midgut, Hindgut, and Unknown Primary. Neuroendocrinology 2012;95:157-76. [Crossref] [PubMed]
- Ramage JK, Davies AH, Ardill J, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours. Gut 2005;54 Suppl 4:iv1-16. [Crossref] [PubMed]
- Wu L, Fu J, Wan L, et al. Survival outcomes and surgical intervention of small intestinal neuroendocrine tumors: a population based retrospective study. Oncotarget 2017;8:4935-47. [PubMed]
- Ahmed A, Turner G, King B, et al. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer 2009;16:885-94. [Crossref] [PubMed]
- Hellman P, Lundström T, Ohrvall U, et al. Effect of surgery on the outcome of midgut carcinoid disease with lymph node and liver metastases. World J Surg 2002;26:991-7. [Crossref] [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program () SEER*Stat Database: Incidence - SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2016 Sub (2000-2014)
- Linked To County Attributes - Total U.S., 1969-2015 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2017, based on the November 2016 submission.www.seer.cancer.gov - Randle RW, Ahmed S, Newman NA, et al. Clinical outcomes for neuroendocrine tumors of the duodenum and ampulla of Vater: a population-based study. J Gastrointest Surg 2014;18:354-62. [Crossref] [PubMed]
- StataCorp 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.
- R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/
- Norlén O, Stålberg P, Zedenius J, et al. Outcome after resection and radiofrequency ablation of liver metastases from small intestinal neuroendocrine tumours. Br J Surg 2013;100:1505-14. [Crossref] [PubMed]
- Watzka FM, Fottner C, Miederer M, et al. Surgical Treatment of NEN of Small Bowel: A Retrospective Analysis. World J Surg 2016;40:749-58. [Crossref] [PubMed]
- Citterio D, Pusceddu S, Facciorusso A, et al. Primary tumour resection may improve survival in functional well-differentiated neuroendocrine tumours metastatic to the liver. Eur J Surg Oncol 2017;43:380-7. [Crossref] [PubMed]
- Landerholm K, Zar N, Andersson RE, et al. Survival and prognostic factors in patients with small bowel carcinoid tumour. Br J Surg 2011;98:1617-24. [Crossref] [PubMed]
- Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg 2000;190:432-45. [Crossref] [PubMed]
- Sarmiento JM, Heywood G, Rubin J, et al. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg 2003;197:29-37. [Crossref] [PubMed]
- Sadowski SM, Neychev V, Millo C, et al. Prospective study of 68Ga-DOTATATE positron emission tomography/computed tomography for detecting gastro-entero-pancreatic neuroendocrine tumors and unknown primary sites. J Clin Oncol 2016;34:588-96. [Crossref] [PubMed]