Personalised approach in combined treatment of cholangiocarcinoma: a case report of healing from cholangiocellular carcinoma at stage IV
Introduction
Cholangiocellular carcinoma (CCA) is a highly aggressive tumor. It is diagnosed in 3% of patients with gastrointestinal tract tumors (1). In western European countries and in Russia incidence of CCA is not higher than 2–3/100,000 of population (2,3). More often, CCA is encountered in Asian countries where disease rate as high as 30/100,000 of population. Main reason is infection with liver flukes: Opisthorchis viverrini, O. felineus, and Clonorchis sinensis (4). Primary sclerosing cholangitis which is associated with inflammatory bowel diseases is also a risk factor for CCA. Incidence of CCA at the patients with primary sclerosing cholangitis is 10% (5).
The mainstay in CCA treatment is still surgical resection which is possible in 10–40% cases (6). After the radical surgical resection recurrence rate in the first 2 years is 60% (7). Five-year survival rate is from 15% to 40% (8). In cases of advanced disease only chemo-/chemoradiotherapy is possible [Table 1: chemotherapy for cholangiocarcinoma (9,10)].
The big hopes are being made on targeted therapy in the future. For implementation of immune therapy to the patients it is necessary to hold genetic sequencing of tumor to determine mutations in cells which allow to use the best targeted therapy. The problem in CCA targeted therapy is a diversity of genetical mutations. Nevertheless, some monoclonal antibodies are already utilized in treatment, for example: lapatinib, sorafenib, selumetinib.
Case presentation
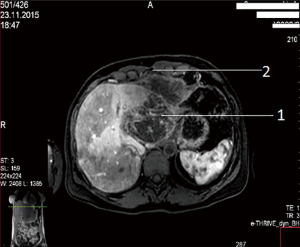
A female patient of 38 years is seen in November of 2015 complaining on pain in right upper quadrant of abdomen. MRI revealed CCA, which occupied segments 2–3 (8 cm × 12 cm × 9 cm) with anterior abdominal wall, diaphragm and pericardial invasion (T4NxM0) (Figure 1: MRI before resection).
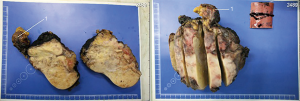
In order to reduce vascularization of tumor, 12 days prior to surgery endovascular embolization of branches of left lobe was performed. In December 2015, the patient had undergone an operation. Tumor occupying segments 2–3 of liver with size 10 cm × 8 cm with anterior abdominal wall, diaphragm and pericardium invasion was detected. Lymph nodes of hepatoduodenal ligament were also involved (Figure 2: intraoperative view).
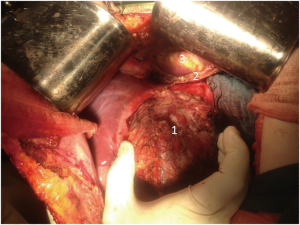
Segments 2–3 were removed with pericardial resection, lymphatic dissection of hepatoduodenal ligament, cholecystectomy and involved anterior wall was also resected (Figure 3: resected tumor). Pericardium was sewed with interrupted sutures and drained. The postoperative period was uneventful and the patient was discharged 2 weeks after surgery.
Immunohistochemistry revealed: low differentiated CCA of left liver lobe (12 cm × 7 cm × 13 cm) with perineural and intravascular invasion, involving hepatic capsule and R1 resection border, penetrating into diaphragm and pericardium. pT4 pN1(3/6) cM0; M8160/3, grade 3; R1; Pn1; L1, V1.
Tumor cells had expression of:
- Cytokeratin 7 (clone OV-TL 12/30, Cell Marque)—pronounced (+++);
- Ki67 (clone SP6, Cell Marque)—nuclear (+++) approximately in 40% of tumor cells;
- Beta-Catenin (clone 14, Cell Marque)—moderate (++) membrane-cytoplasmic.
Tumor cells are negative for:
- Hep-Par1 (clone OCH1E5, Cell Marque);
- Chromogranin A (clone DAK-A3, DAKO);
- Synaptophysin (clone MRQ-40, Cell Marque).
In January of 2016, i.e., after a month of surgery progression of tumor rise was revealed on positron emission tomography (PET). Patient received 2 courses of GEMOX chemotherapy by the following schedule: 1st day—gemcitabine 1,400 mg intravenous (IV), 2nd day—oxaliplatin 200 mg IV, 8th day—gemcitabine 1,400 mg IV.
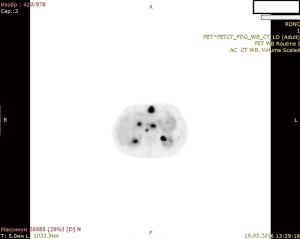
After 2 courses of GEMOX therapy PET showed negative dynamic, so chemotherapy was ceased (Figure 4: PET. Negative dynamic after chemotherapy).
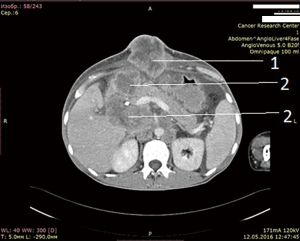
After unsuccessful chemotherapy it was decided to start patient on Pembrolizumab. But this drug also didn’t give any improvement. In April 2016, i.e., after 5 months after surgery, patients developed mechanical jaundice due to choledochal obstruction with metastatic lymph nodes from hepatoduodenal ligament. To release the jaundice endoscopic choledochal stenting had been performed. In May 2016 metastases to hepatoduodenal ligament lymph nodes, retroperitoneal lymph node metastases and metastases to lungs, soft tissues of arms and foots, ascites were detected (Figure 5: abdominal CT. Six months after operation, after 2 courses chemotherapy and 2 courses pembrolizumab).
Next generation sequencing was performed which revealed BRAF V600E mutation in tumor cells. Having taken in account the results of genetic sequencing and the availability of specific inhibitors of this gene, we decided to start our patient on dabrafenib 300 mg pro day + trametinib 2 mg/day.
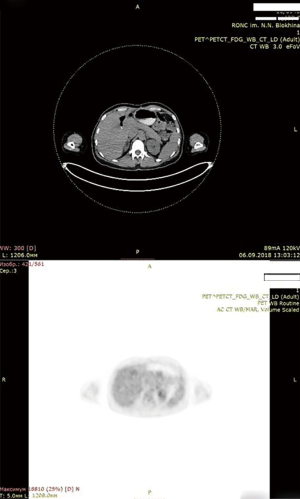
Soon after months of initiation of targeted therapy we achieved the first positive result. After 7 months full response with complete tumor resolution was achieved. Patient is under thorough control and she is free from tumor according to her latest PET scan on December 2018. To our knowledge this case report is longest remission on dabrafenib + trametinib in CCA patients. Achieved remission lasts for 28 months (Figure 6: thirty-four months after the operation, 28 months after initiation of targeted therapy with dabrafenib + trametinib. No active tumor sites).
Discussion
Genes, in which mutations play a role in development of CCA are genes-regulators of genomic stability, controllers of cell cycle, genes responsible for Wnt signaling, cytokine signaling, TGF-beta signaling, MAPK, AKT/PI3K and other forms of conveying stimulus from cell surface to its nucleus (11).
The most frequent mutation in CCA is activating KRAS mutation which is encountered in 50% of cases. KRAS gene is a regulator of cell proliferation. Mutation in this gene leads to loose of self-inhibition resulting in constant division of cells and finally in tumor formation (11).
Second most common mutation is TP53 gene mutation (up to 40% of cases). This gene is responsible for protein p53 synthesis, which regulates cell division and keeps them from over proliferation. Protein p53 is also known as gene guardian because it initiates apoptosis in cells with mutated DNA. Mutation in gene TP53 leads to loss of protein p53 and all his protective functions.
BRAF, NRAS, PI3K, EGFR and MET are rare mutations with total incidence <5% (11).
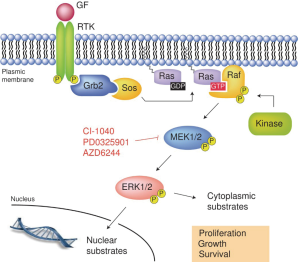
BRAF gene encodes protein B-raf which is a protein from Raf kinases and conveys signals from cell surface to its nucleus. This process is carried out through Ras-Raf-MEK-MAPK kinase pathway. Receptor on cell surface is tyrosine kinase receptor and ligand of this receptor is growth factor, cytokines, hormones. Thus, BRAF gene regulates cell proliferation, differentiation, migration and apoptosis [Figure 7: tyrosine kinase receptor and Ras-Raf-MAP kinase pathway (12)].
In cases of inherited BRAF gene mutation patients are encountered with cardiofaciocutaneous syndrome which manifests with heart malformations, malformations of face, cutaneous and central nervous system (CNS) problems. In cases of acquired BRAF gene mutation patients suffer from different tumors: non-Hodgkin lymphoma, colorectal cancer, malignant melanoma, papillary thyroid cancer, non-small cell lung cancer, glioblastoma and astrocytoma of brain.
More than 30 mutations in BRAF gene were registered which have oncogenic potential. Mostly codon V600 affected, there amino acid substitution occurs which eventually leads to B-raf protein becoming constantly active. In 90% of cases valine (V) is a substitute for glutamic acid (E) that’s why mutations are called V600E (13).
Mutation in BRAF V600E can lead to CCA from 0% to 22% of cases (14).
Dabrafenib is B-raf protein inhibitor which is in mutated cells, is in constant hyperactive form. This leads to more often signaling through Ras-Raf-MEK-MAPK kinase pathways and cells hyperproliferate. Inhibition of B-raf causes blockage in Ras-Raf-MEK-MAPK kinase pathway.
Implementation of dabrafenib as monotherapy leads to development of resistance after 6–7 months. To prevent resistance dabrafenib is used in conjunction together with trametinib, a MEK inhibitor, which also blocks Ras-Raf-MEK-MAPK kinase pathway (15).
This combination was first described for melanoma treatment. There are few cases of CCA treatment with BRAF V600E mutation with good results in literature. In one case the patient achieved full response which lasted 9 months, but then recurrence happened. Second patient gave partial response after 2 months from initiating targeted therapy and it lasts 5 months (16). In another report a partial response was achieved and a cease of progression during 6 months (17). There is also a case report of partial response which lasted 8.5 months (18).
In Russian literature we didn’t find any cases of CCA with mutation in BRAF V600E.
Conclusions
Our clinical case completes rare cases of successful treatment of CCA with BRAF V600E and opens new horizons and opportunities in treatment of these type of tumors. It is very important to make a personalized approach to the treatment.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- de Groen PC, Gores GJ, LaRusso NF, et al. Biliary tract cancers. N Engl J Med 1999;341:1368-78. [Crossref] [PubMed]
- Bridgewater JA, Goodman KA, Kalyan A, et al. Biliary Tract Cancer: Epidemiology, Radiotherapy, and Molecular Profiling. Am Soc Clin Oncol Educ Book 2016;35:e194-203. [Crossref] [PubMed]
- Breder VV. Rak zhelchevyvodyashchej sistemy. Prakticheskaya onkologiya 2012;13:269-75.
- Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol 2008;24:349-56. [Crossref] [PubMed]
- Lazaridis KN, Gores GJ. Primary sclerosing cholangitis and cholangiocarcinoma. Semin Liver Dis 2006;26:42-51. [Crossref] [PubMed]
- Cidon EU. Resectable Cholangiocarcinoma: Reviewing the Role of Adjuvant Strategies. Clin Med Insights Oncol 2016;10:43-8. [Crossref] [PubMed]
- Gil E, Joh JW, Park HC, et al. Predictors and patterns of recurrence after curative liver resection in intrahepatic cholangiocarcinoma, for application of postoperative radiotherapy: a retrospective study. World J Surg Oncol 2015;13:227. [Crossref] [PubMed]
- Shimada K, Sano T, Nara S, et al. Therapeutic value of lymph node dissection during hepatectomy in patients with intrahepatic cholangiocellular carcinoma with negative lymph node involvement. Surgery 2009;145:411-6. [Crossref] [PubMed]
- Benson AB III, D’Angelica MI, Abbott DE, et al. Hepatobiliary Cancers, Version 1.2017: Featured Updates to the NCCN Guidelines. J Natl Compr Canc Netw 2017;15:563-73. [Crossref] [PubMed]
- Kovalenko Y, Kukeev A, Zharikov A, et al. Role of adjuvant drug therapy in combined treatment for cholangiocellular carcinoma. Vopr Onkol 2018;64:171-4.
- Gibiino G, Fabbri C, Fagiuoli S, et al. Defining the biology of intrahepatic cholangiocarcinoma: molecular pathways and early detection of precursor lesions. Eur Rev Med Pharmacol Sci 2017;21:730-41. [PubMed]
- Frémin C, Meloche S. From basic research to clinical development of MEK1/2 inhibitors for cancer therapy. J Hematol Oncol 2010;3:8. [Crossref] [PubMed]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949-54. [Crossref] [PubMed]
- Chong DQ, Zhu AX. The landscape of targeted therapies for cholangiocarcinoma: current status and emerging targets. Oncotarget 2016;7:46750-67. [Crossref] [PubMed]
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694-703. [Crossref] [PubMed]
- Lavingia V, Fakih M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J Gastrointest Oncol 2016;7:E98-102. [Crossref] [PubMed]
- Kocsis J, Árokszállási A, András C, et al. Combined dabrafenib and trametinib treatment in a case of chemotherapy-refractory extrahepatic BRAF V600E mutant cholangiocarcinoma: dramatic clinical and radiological response with a confusing synchronic new liver lesion. J Gastrointest Oncol 2017;8:E32-8. [Crossref] [PubMed]
- Loaiza-Bonilla A, Clayton E, Furth E, et al. Dramatic response to dabrafenib and trametinib combination in a BRAF V600E-mutated cholangiocarcinoma: implementation of a molecular tumour board and next-generation sequencing for personalized medicine. Ecancermedicalscience 2014;8:479. [Crossref] [PubMed]