Current and future systemic treatment options in metastatic pancreatic cancer
Introduction
Pancreatic adenocarcinoma is the fourth most-frequent cause of tumor related death in western world (1). Median survival is 4 to 6 months and median 5-year survival is less than 5% (2). Great majority of the patients with pancreatic adenocarcinoma presents at advanced stage, either with metastatic or locally advanced disease. Actuarial 5-year survival rate for early stage operable disease with adjuvant treatment is around 20% (3,4). However, 70% of them recurs and need palliative treatment. Standard treatment of metastatic and locally advanced pancreatic cancer patients who cannot be treated with chemoradiation or surgery is chemotherapy. Pancreatic cancer is a well-known relatively chemo-refractory disease. Evidence changed in recent years from single agent gemcitabine treatment to combination regimens. FOLFIRINOX and gemcitabine plus nab-paclitaxel became two standard options for metastatic pancreatic cancer for patients with good performance status (5,6). Targeted agents, immunotherapy and vaccines are the most popular fields of clinical trials in advanced pancreatic cancer and we will reach a bulk of new clinical data in the treatment of metastatic pancreatic cancer in near future.
Cytotoxic therapy
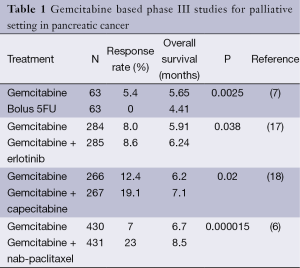
Cytotoxic chemotherapy is the standard treatment option for metastatic and locally advanced pancreatic cancer patients cannot be treated with surgery or radiochemotherapy. Chemotherapy trials had been failed to show benefit for a long time in the past. In 1997 gemcitabine monotherapy became the standard treatment with the landmark study by Burris et al. (7). Gemcitabine (n=63) monotherapy was compared with weekly blous 5-fluorouracil (n=63) and modest survival benefit was shown in gemcitabine group (5.6 vs. 4.4 months). But clinical benefit was evident regarding performance status and pain control in gemcitabine group. Gemcitabine was used as the standard treatment for many years due to good patient tolerance and improved quality of life in metastatic cancer patients. Several agents including capecitabine, irinotecan, oxaliplatin and cisplatin were tested in combination with gemcitabine but survival benefit could not be shown in any of those phase III studies (8-11). Several targeted agents were also studied in combination with gemcitabine in phase II or III trials. Studies of vismodegib, masitinib, sorafenib, AMG479 and IPI926 in combination with gemcitabine failed to show survival benefit (12-16). Significant phase III studies of gemcitabine were summarized in Table 1. A small survival benefit was shown with platinum derivatives and capecitabine when added to gemcitabine in meta-analyses due to underpowered studies to show small differences (18,19). Additional toxicity came with this marginal survival benefit with gemcitabine and platinum or capecitabine combinations.
Full table
Combination therapy for advanced pancreatic cancer was controversial until year 2011. Prodige 4-ACCORD 11 randomized phase III trial compared FOLFIRINOX regimen with gemcitabine in good performance status, 336 untreated, metastatic pancreatic adeno cancer patients (5). Inclusion criteria were strict as permitting patients up to age of 75 years, with ECOG performance status 0 or 1, nearly normal bilirubin, good bone marrow and renal function, and without a history of heart disease. This study met the primary endpoint of OS as 11.1 vs. 6.8 months in FOLFIRINOX and gemcitabine arms, respectively (HR=0.57, P=0.0001). ORR (31.6% vs. 9.4%, P=0.0001) and PFS (6.4 vs. 3.3 months, P=0.001) was also superior in FOLFIRINOX compared to gemcitabine group consistent with OS results. These better survival rates and responses came with the expense of excess toxicity. Febrile neutropenia (5.4% vs. 0.6%, P=0.009), thrombocytopenia (9.1 vs. 2.4, P=0.008), peripheral neuropathy (9% vs. 0%, P=0.001), vomiting (14.5% vs. 4.7%, P=0.002), diarrhea (12.7 vs. 1.2, P=0.0001), thromboembolic events (6.6% vs. 4.1%) and growth factor support (42.5% vs. 5%) rates were higher in FOLFIRINOX compared to gemcitabine group. But elevated LFTs were higher in gemcitabine group (20.8% vs. 7.3%). FOLFIRINOX combination regimen was approved for the first line treatment of metastatic pancreas adenocarcinoma patients with good performance status regarding results of this trial.
Chemoresistance of pancreatic cancer is partly attributed to stroma rich characteristic of the tumor. Albumin-bound paclitaxel (nab-paclitaxel) was shown to bind to protein SPARC (secreted protein acidic and rich in cysteine) also known as osteonectin, which is overexpressed by fibroblasts in the pancreatic cancer microenvironment (20,21). Thus nab-paclitaxel renders an effective amount of cytotoxicity by depleting tumor stroma. The molecular mechanism of nab-paclitaxel is not fully understood and simply albumin avidity of tumor cells might deliver a high concentration of chemotherapeutic in the tumoral tissue. Nab-paclitaxel came as another combination option with gemcitabine for patients with advanced stage pancreatic cancer. After the impressive response rate (48%) and survival of 12 months from the phase I-II trial, phase III trial was conducted (22). The MPACT trial compared gemcitabine plus nab-paclitaxel with gemcitabine in 861 untreated metastatic pancreatic adeno cancer patients (6). This study also met the primary endpoint of OS and nab-paclitaxel was the first agent showed OS increment with addition to gemcitabine (8.5 vs. 6.7 months, HR=0.72, P=0.000015). One year survival rate (35% vs. 22%), PFS (5.5 vs. 3.7) and ORR (23% vs. 7%) were higher in gemcitabine plus nab-paclitaxel compared to gemcitabine group. Toxicity related deaths were similar in groups (4% for each) but grade 3-4 neutropenia (38% vs. 20%), fatigue (17% vs. 7%), neuropathy (17% vs. <1%) were higher in combination group. In the subgroup analyses patients with poorer performance status (KPS 70-80) and more bulky disease (liver metastases, >3 metastatic sites and >59XULN CA19.9 level) much benefited from the gemcitabine plus nab-paclitaxel combination regimen.
Treatment selection
Decision of two new standard options for metastatic pancreatic adenocarcinoma might be given according to age (number of patients >70 was lower in Prodige4 ACCORD 11 trial), performance status (MPACT trial consisted a broader spectrum for performance status; KPS 70-100), patients preference of treatment routes and frequency (46 hours infusional 5-fluorouracil vs. weekly nab-paclitaxel treatment) and toxicity profiles (increased hematologic toxicity, febrile neutropenia, diarrhea, fatigue and growth factor support need in FOLFIRINOX regimen and alopecia in nab-paclitaxel combination treatment). Patients who will not tolerate the FOLFIRINOX combination chemotherapy or who do not want a central access might be good candidates for gemcitabine plus nab-paclitaxel study. However gemcitabine monotherapy must be kept in mind as the oldest standard for patients cannot receive FOLFIRINOX or nab-paclitaxel.
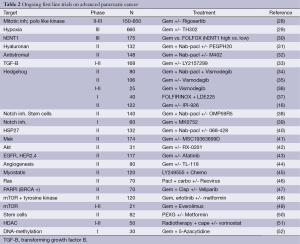
Drug sensitivity model for gemcitabine, irinotecan, oxaliplatin, nab-paclitaxel, 5-fluorouracil and oxaliplatin was generated with pharmacogenomic studies in pancreatic cancer cell lines according to genetic expression of molecular pathways i.e., the transforming growth factor B (TGF-B), hedgehog and jak-stat (8,23-26). Sangar et al. validated this pharmacogenomic test in a phase II trial in pancreatic adenocarcinoma patients (n=20) and patients sensitive to drug had longer TTP compared to intermediate sensitive and resistant patients (7.3 vs. 5.3 vs. 3.7 months) according to pharmacogenomic analysis (27). Pharmacogenomic test was shown to be predictive for treatment efficacy regarding TTP. Future studies with this pharmacogenomic tests might help 1st and 2nd line treatment decisions and treatment choice of nab-paclitaxel plus gemcitabine or FOLFIRINOX as the 1st line treatment. A high SPARC expression is associated with improved response to nab-paclitaxel and pre-treatment pharmacogenomic testing of SPARC might be useful for choosing patients for gemcitabine plus nab-paclitaxel treatment (22). There are a number of ongoing trials mostly with gemcitabine chemotherapy backbone on the first line treatment of advanced pancreatic cancer listed in Table 2 and a third treatment option might come from these trials.
Full table
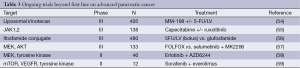
Data on second line treatment of metastatic pancreatic cancer is sparse. The only second line, randomized phase III study in advanced pancreatic cancer tested FOLFOX versus best supportive care after first line treatment with gemcitabine failure. This study demonstrated a median second line survival benefit of 4.82 months compared to 2.30 months with best supportive care (53). That might be a good option in fit patients progressed on gemcitabine treatment. In FOLFIRINOX trial 47% of the patients were treated with second line therapy and most of them received gemcitabine (5). Thus gemcitabine might be an option patients progressed on FOLFIRINOX treatment. Ongoing trials for the second line treatment of advanced pancreatic cancer are summarized in Table 3.
Full table
Targeted therapy
During last 10 years various targeted agents were tested alone or in combination with gemcitabine for treatment of advanced pancreatic cancer. But all but one failed to improve patients’ survival significantly. Erlotinib, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor was the first agent achieved survival benefit when added to gemcitabine (17). However the difference was minimal (6.24 vs. 5.91 months, P=0.038) and raised the question of clinical significance. A prolonged survival of 10.5 months was seen in a subgroup of patients who developed grade 2 or severe skin rash. Skin rash was the most important adverse effect. Skin rash was proposed as a predictive marker for erlotinib benefit (60). However it is not clearly defined as a predictive tool. The EGFR monoclonal antibody cetuximab and VEGF antibody bevacizumab were failed in phase III studies of advanced pancreatic cancer (61,62). Another EGFR monoclonal antibody nimotuzumab in combination with gemcitabin had shown better overall survival compared to gemcitabine plus placebo (8.7 vs. 6.1 months) with tolerable toxicity in a recent phase II trial in first line treatment of locally advanced or metastatic pancreas cancer patients (63). Other members of small molecule tyrosine kinase inhibitors axitinib, sorafenib and tipifarnib (a farnesyl transferase inhibitor) with combination of gemcitabine were compared with single agent gemcitabine in different phase III trials. But they were also failed to show any benefit in treatment of advanced pancreatic cancer (64-66). Masitinib a c-kit inhibitor of mast cell function, marimastat an agent against secreted matrix proteases were also tested in phase III randomized trials with or without gemcitabine. However no survival benefit was seen with adding these agents to gemcitabine in advanced pancreatic cancer (67,68).
Insulin like growth factor 1 receptor (IGF1R) is highly expressed in pancreatic cancer and takes role in downstream signalling cascades for cancer cell survival and proliferation thorough a KRAS-dependent and independent pathway. It was another target for drug development for several solid tumors and also pancreatic cancer. However IGF1 R inhibitor AMG-479 and monoclonal antibody cixutumumab failed to show a survival benefit (15,69).
K-ras is a major driver in pancreatic cancer and mutated in 90% of the cases. It causes an uncontrolled activity of downstream pathway of raf, MEK and ERK, leading to tumor cell proliferation and survival. Mitogen activated protein kinase MEK is an important druggable target in pancreatic carcinoma in which activating K-ras mutation is seen frequently. Trametinib (GSK1120212) a MEK inhibitor failed to show survival benefit when added to gemcitabine in advanced pancreatic cancer (70). Another MEK inhibitor MSC1936369B is being tested in combination with gemcitabine in first line treatment of advanced pancreatic cancer in a phase II trial (41). A phase II study is evaluating another MEK inhibitor AZD6244 in combination with tyrosine kinase inhibitor erlotinib in second line treatment of advanced pancreatic cancer (58).
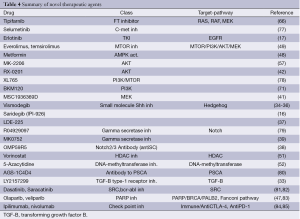
The PI3K/Akt and mTOR pathway takes role in tumor cell proliferation, survival and metabolism is another therapeutic target in advanced pancreatic cancer. Increased activity of PI3K/Akt and mTOR pathway might take an important role in resistance of drugs effecting ras-raf-MEK and ERK pathway. A phase II study of an Akt antisense oligonucleotide, RX-0201 in combination with gemcitabine is completed and results are awaited (42). A study of PI3K inhibitor BKM120 in combination with mFOLFOX-6 regimen in advanced stage solid tumors including pancreatic cancer is going on (71). The BEZ235 is a combined inhibitor of PI3Kand mTOR. A phase I study of BEZ235 in combination with the MEK inhibitor MEK162 with the strategy of hitting two pathways at the same time is completed in advanced solid tumor patients carrying K-ras, Nras and/or Braf mutations including pancreatic cancer and results are awaited (72). The study of mTOR inhibitor everolimus monotherapy by Wolpin et al. had shown a PFS and OS of 1.8 and 4.5 months respectively in gemcitabine refractory pancreatic cancer patients (73). Another phase II study of everolimus in combination with erlotinib in previously treated advanced pancreatic cancer patients was terminated due to futility and significant adverse effects (74). A phase II trial of the other mTOR family member temsirolimus is completed in locally advanced or metastatic pancreas cancer patients and results are pending (75). A phase I and II combination study of everolimus and sorafenib in advanced solid tumor patients including pancreas cancer refractory to gemcitabine was completed and results are also pending (76). A phase I/II study of everolimus in combination with gemcitabine in advanced pancreas cancer patients is completed and results are awaited (49). A list of novel therapeutic targets and drugs is given in Table 4.
Full table
A commonly used oral antidiabetic drug metformin was shown to activate adenosine monophosphate-activated protein kinase (AMPK).The AMPK inhibits mTOR pathway by phosphorylation and stabilization of the tumor suppressor gene TSC2 (86). One of the mechanisms for TKI-resistance is hyperactivation of mTOR pathway. Blocking the mTOR pathway might be a good strategy for overcoming TKI resistance. A phase II randomized study of metformin in combination with erlotinib and gemcitabine compared to placebo in advanced pancreatic cancer patients is going on (48).
Novel therapeutics
Pancreas cancer has an extensive stromal tissue which is a unique histological feature. This dominant desmoplastic tissue might contribute the weak penetration of the applied drugs and act as a protective barrier from the treatments. It was hypothesized as a chemoresistance mechanism of pancreas carcinoma (87). Sonic hedgehog pathway takes an important role for stimulating stromal reaction. Vismodegib an hedgehog inhibitor is the first drug approved in advanced and metastatic basal cell skin carcinoma (88). Various clinical trials of vismodegib in combination with gemcitabine and gemcitabine plus nab-paclitaxel are ongoing in recurrent or advanced pancreatic cancer patients (34-36). Another hedgehog inhibitor IPI-926 or placebo in combination with gemcitabine is studied in a phase II randomized study in metastatic pancreas cancer patients (16). This study is completed and results are pending. Hedgehog inhibitor LDE225 is tested in combination with FOLFIRINOX in untreated advanced pancreatic cancer patients and the study is ongoing (37).
The Notch pathway is thought to take role in pancreas carcinogenesis and Notch ligand and receptor are shown to be highly expressed in pancreas cancer (89,90). OMP-59R5 is a fully human monoclonal antibody that targets the Notch2 and Notch3 receptors. It downregulates Notch pathway signaling and affects pericytes, tumor stroma and microenvironment and thought to have anti-cancer stem cell effect. The ALPINE trial testing OMP-59R5 with gemcitabine and Nab-paclitaxel in first-line advanced pancreatic cancer patients showed well tolerability and responses (PR=46%, DCR=77%) in early phase I results (91). Gamma secretase is an enzyme causes proteolytic cleavage and release of the intracellular domain of the Notch and activates Notch signalling pathway. A phase II study of gamma-secretase inhibitor RO4929097 monotherapy is going on in pretreated metastatic pancreas cancer patients (92). Another gamma-secretase inhibitor MK0752 and gemcitabine combination are being tested for first line treatment of stage III and IV pancreas cancer patients (39).
Histone de-acetylation (HDAC) and DNA hypermethylation are two major epigenetic changes cause tumor supressor gene silencing and tumor cell proliferation, growth and progression. Vorinostat, HDAC inhibitor is being tested in locally advanced pancreas cancer patients in combination with capecitabine and radiotherapy in a phase I and II study (51). The chemical cytosine analogue 5-azacitidine inhibits DNA methyltransferase and a phase I study in combination with gemcitabine is going on in first line treatment of advanced pancreatic cancer patients (52).
TGF-B is another regulator pathway of stromal reaction and TGF-B takes role in stimulating stromal reaction, invasion, metastasis and promoting angiogenesis in pancreas cancer (93). Trabedersen, an antisense oligodeoxynucleotide which inhibits TGF-B2 expression was shown to have good efficacy and safety profile in the second line treatment of pancreas cancer patients (n: 37; median OS, 13.4 months) (94). A phase II study of gemcitabine in combination with a specific type 1 receptor inhibitor of TGF-beta, LY2157299 or placebo is recruiting patients (33).
Pancreas cancers are rich of tumor stroma and have a high level of hyaluronan. PEGPH20 degrades hyaluronan, reduces interstitial fluid pressure and facilitates drug delivery (95,96). It has shown to improve efficacy when used with cytotoxics. A phase IB trial of gemcitabine plus PEGPH20 had shown promising efficacy and phase II and III trials of gemcitabine + nabpaclitaxel ± PEGPH20 (HALOZYME) and FOLFIRINOX +/- PEGPH20 (SWOG-NCI) are planned (97,98).
The DNA double-strand breaks (DSBs) are mainly repaired by homologous recombination, a process mediated by BRCA1 and BRCA2 proteins which sustains genomic stability and cell survival (99). Alternative poly (ADP-ribose) polymerase (PARP) pathway takes the main role for DNA repair when BRCA dysfunction occurs. PARP is a critical enzyme of cell proliferation and DNA repair mediates repair of DNA single strand breaks (SSB), and rescues tumor cells from DNA damage. PARP represents a good therapeutic target in BRCA mutated/dysfunctional tumors. Inhibition of PARP-1 activity prevents the recruitment of DNA repair enzymes and leads to failure of SSB repair. DNA single strand breaks accumulate, induce formation of DNA replication fork arrests, and form DSBs (100). In the combined absence of PARP activity and BRCA1 or BRCA2 activity, both repair pathways are disabled; DNA DSBs cannot be repaired properly. DSBs can induce genomic instability and ultimately lead to tumor cell death. PARP inhibitors have shown efficacy in BRCA mutated ovary and breast cancer patients (101-105). A 5% to 7% of pancreatic cancer patients show germline mutations of BRCA 1 or 2. Preclinical data showed susceptibility to alkylating agents and Parp inhibitor in Capan-1 BRCA 2 deficient pancreatic cancer cell line (106). A randomized phase II study of gemcitabine + cisplatin +/- veliparib in BRCA 1-2 and PALP-2 mutated locally advanced or metastatic pancreatic cancer patients is being continued (47). The second part of this trial which is a single arm phase II, is going on in previously treated pancreatic cancer patients. Novel agents on the treatment of advanced pancreatic cancer are summarized in Table 4.
Platinum compounds directly bind to DNA and causes double strand breaks. A dysfunction in BRCA1 and its pathway is associated with a specific DNA-repair defect that sensitizes cells to platinum drugs in animal models (107,108). Platinum compounds showed high responses in triple negative breast cancer which share similar features with BRCA deficient patients (109,110).
Immunotherapies
Immunologic treatments are increasingly studied in last few years in various tumors in medical oncology. Unmet medical need in pancreatic cancer directed researchers to investigate new pancreatic cancer treatments and also immunological approaches. After the first positive results of ipilimumab came from phase III study of metastatic malignant melanoma, interest on immunological treatments increased. Immunologic treatments might be classified as passive immunotherapy approaches as the use of antibodies or in vitro generated effector cells, and vaccination for stimulating antitumoral response. There are different ways of delivering vaccines. Dendritic cell (DC) vaccines combine tumoral antigen with DCs for presenting them to effector T cells. Viral or bacterial DNA is inserted to human cells to modulate cell-mediated immunity by the DNA vaccines. Peptides are inserted to human cells by T-cell receptor peptide vaccines for increasing cell mediated immunological response. DCs are the most potent antigen presenting cells. They can cause a high antigenic response via stimulating T and B cells. Kimura et al. showed DC vaccine plus lymphokine activated killer cell treatment and chemotherapy prolonged overall survival compared to patients received only DC vaccine or chemotherapy (111). Carcinoembryonic antigen (CEA) is an oncofetal antigen that is expressed in epithelial malignancies and pancreatic cancer. It is one of the highly expressed antigens in pancreatic cancer might be used with DCs for vaccine treatment of pancreatic cancer (112). MUC1 is another protein which is highly expressed in pancreatic cancer (113). Phase I and II studies of MUC1 antigen pulsed DC vaccines showed hopeful results in advanced pancreatic cancer (114,115). A phase I study in advanced pancreatic cancer with vaccine containing vaccinia virus expressing CEA and MUC1 and costimulatory molecules showed well tolerability an overall survival advantage in immune responsive patients (116). But a phase III trial of fowlpox viruses expressing CEA and MUC1 and costimulatory molecules failed to improve overall survival when compared to chemotherapy or best supportive care in palliative setting in pancreatic cancer patients (117). Heat shock proteins are a family of chaperone proteins expressed in all species which are induced by stress conditions. They are presented within HLA class I complex on the cell surface. HSPPC-96 is a HSP-based vaccine used in a small study of resected pancreas cancer patients with tolerable toxicity profile and long survival durations in some patients (118).
Algenpantucel-L is an irradiated, live combination of two human allogeneic pancreatic cancer cell lines. These cells express the murine enzyme alpha-1,3-galactosyl transferase (alpha-GT) which directs the synthesis of alpha-galactosyl epitopes on surface proteins and glycolipids of such cell lines. Alpha-Ga1 epitopes are absent in humans but large amount of alpha-Ga1 antibodies exists (119). Alpha-Ga1 antibodies and alpha Ga1 epitopes in algenpantucel–L activates complement mediated lysis and antibody dependent cell mediated toxicity against algenpantucel-L cells (120). Phase II adjuvant study of algenpantucel in combination with radiation plus 5-fluorouracil and gemcitabine treatments in resected pancreatic cancer patients reached a one year DFS of 62% and OS of 86% meeting primary and secondary endpoints (121). This promising result in the adjuvant setting was one of the important factors directing researchers’ focus on vaccine trials in pancreatic cancer. Granulocyte monocyte colony stimulating factor (GM-CSF) is a potent cytokine able to mobilize monocytes, eosinophils and lymphocytes to the tumor sites. Early studies have shown the efficacy of GM-CSF vaccine in resected pancreatic cancer patients and trials in metastatic pancreas cancer with GM-SCF are ongoing (122).
K-ras mutations are found in up to 90% of pancreatic cancers (123). K-ras mutation is specific for tumor cells and is not present in normal cells. These mutations can be targets for a specific T cell mediated toxicity. A phase I/II trial of synthetic mutant ras peptides with GM-CSF showed a prolonged survival in immune responders compared to nonresponders (5 vs. 2 months) in advanced pancreatic cancer patients (124). Median survival was also longer for also immune responders among resected pancreatic cancer patients (Median OS: 20% vs. 0%, for 10 years).
Telomerase is a ribonucleotide enzyme that is expressed in almost all of the cancer but not in normal cells (125). Telomerase maintains telomers which exist at the end of the chromosomes and elicits stability. It is generally activated in cancer cells and was shown to be expressed in pancreatic cancer (126). A telomerase peptide vaccine GV1001 with GM-CSF was shown to prolong survival in unresectable pancreatic cancer patients in a phase I-II study (127). However, phase III study of GV1001 with gemcitabine sequential combination versus gemcitabine was closed due to lack of survival advantage in unresectable pancreas cancer patients (128,129). Another phase III study of capecitabine plus gemcitabine with or without GM-CSF plus GV1001 in locally advanced or metastatic pancreatic cancer patients was completed and results are awaited (130). Ongoing phase II vaccine trials are summarized in Table 5.
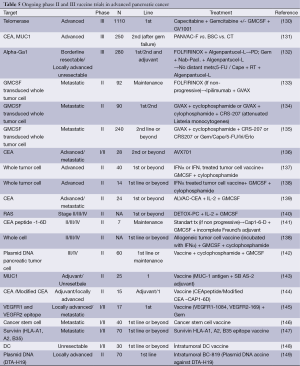
Full table
Pancreatic cancer is one of the immunologically quiescent tumors. Effector T cell infiltration is not a natural response for pancreatic cancer. But immune system can be provoked. Gemcitabine plus CD40 agonist activating T cells has been shown to reduce tumor burden in advanced pancreatic cancer patients in a phase I study (150). Zheng et al. studied a vaccine with or without intravenous low dose or oral metronomic cyclophosphamide in pancreatic cancer patients in a three arm neoadjuvant and adjuvant study (151). Cyclophophamide was used to deplete regulatory T cells. Intratumoral and peritumoral lymphoid aggregates were found in surgical specimens of the vaccinated patients (152). Lymphoid aggregates in pancreatic adenocarcinomas consisted organized T and B cell zones and germinal center like structures. PD-L1 expressing and PD-1 positive cells were upregulated in lymphoid aggregates but not in pancreatic adenocarcinomas without T cell infiltration. Vaccines can induce tumor infiltrating lymphocytes in non-immunogenic tumors. These tumor infiltrating lymphocytes can secrete IFN-gamma and other cytokines that up-regulate PD-1 and PD-L1 pathway. But vaccine induced T cells might be downregulated by the suppressive mechanisms within the tumor. Thus vaccines must be given with agents modulate these suppressive mechanisms and activate T cell response. Anti-PD-1 antibody was shown to enhance infiltration of vaccine induced tumor specific infiltrating lymphocytes active against mesothelin epitope in a preclinical pancreatic cancer model (153).
Modulating regulatory pathways might be another strategy to enhance vaccine’s efficacy in pancreatic cancer. Ipilimumab an anti-CTLA4 antibody (Four, 3 weekly, 10 mg/kg induction doses and maintenance q 12 weeks if stable disease or better response is seen at week 22) was given alone or with vaccine to metastatic pancreatic cancer patients in a phase 1B study (154). Thirty metastatic pancreatic patients received two or more lines of chemotherapy were included to this study. Overall survival was longer in ipilimumab + GVAX than ipilimumab alone treated patients (5.5 vs. 3.3 months). Twelve month OS and response rate was also higher in the combination arm (27% vs. 7% and 45% vs. 0%, respectively). Survival was found to be correlated with CD8+, mesothelin specific T cell quantity. Phase II study of this protocol is under development due to this promising result. Targeting more than one checkpoint pathway at the same time might be another option for getting increased efficacy. Anti-PD-1 agent nivolumab and anti-CTLA-4 agent ipilimumab was given concomitantly to malignant melanoma patients and a higher response with the cost of increased toxicity was seen compared to response rate in single agent ipilimumab studies (40% vs. 32% for responses and 14% vs. 51% for grade 3-5 toxicity) (155,156). Regarding the low amount of T cells in pancreatic cancer microenvironment, combining these immune checkpoint pathway modulators might not be a beneficient strategy due to increased toxicity. Listeria monocytogenes, peptide, DNA, and DC based vaccines are the new vaccines might induce T cells better. Vaccines and immune checkpoint inhibitors as anti-CTLA-4 plus GVAX and anti-PD-1 plus GVAX prime/Listeria boost are the emerging combination strategies. Targeting methylation might unchain the anti-inflammatory signals with hypomethylating strategy and combination with immune checkpoint inhibitors might increase the efficacy. Engineered T cells targeting pancreatic cancer antigens is another emerging era of treatment in advanced pancreatic cancer.
Combining two vaccines might be another strategy to enhance efficacy. GVAX is a DC vaccine which is exposed to whole pancreatic cancer cell irradiated and incubated with GMCF. CRS 207 is a Listeria based vaccine in which a tumor specific antigen mesothelin is incorporated to the Listeria’s chromosome and of which two virulence genes (actA, inlB) were deleted. Listeria is an intracellular microorganism and it secretes and expresses tumor antigens inside the antigen presenting cells. Induction of robust innate and antigen specific adoptive immunity occurs by this way. GVAX alone or in combination with CRS207 was given to advanced pancreatic cancer patients (2 to 1 randomization; n=90) who have failed or refused previous chemotherapy (85). Median OS was higher in combination compared to GVAX alone arm (6.1 vs. 3.9 months, P=0.0172, HR=0.59). Overall survival benefit was more clear among patients treated as 3rd line (5.7 vs. 3.9 months, P=0.0003, HR=0.29). Immunotherapy might be synergistic with different combinations of treatment i.e. chemotherapy and targeted agents.
A randomized phase II study of gemcitabine with or without AGS-1C4D4, a fully human monoclonal antibody to prostate stem cell antigen (PSCA) showed better 6-month survival rates in combination (n=133; 60.9%) versus gemcitabine arm (n=63; 44.4%) in metastatic pancreatic cancer (157). Median survival was and response rate were also higher in the combination group (7.6 vs. 7.6 months and 21.6% vs. 13.1%, respectively). The 6-month SR was higher in PSCA-positive subgroup (79.5% vs. 57.1%).
Immunotherapy might be a promising treatment option for pancreatic cancer. Immunologic treatments have no potential side effects like conventional chemotherapeutics have unique toxicity profile like autoimmune phenomena. There is no phase III data of immunological treatment showing benefit in metastatic pancreas cancer. Absence of pancreatic cancer cell specific antigen and immunological quiescent microenvironment of pancreas cancer are difficulties for investigations on immunologic treatment approaches. Combinations of active and passive immunologic treatments, targeted agents and conventional chemotherapies might be important strategies for increasing efficacy.
In conclusion, FOLFIRINOX and gemcitabine + Nab-paclitaxel are new standard combinations in frontline setting. However they can be integrated to all disease settings in clinical practice. Gemcitabine + nab-paclitaxel combination seems to be more tolerable and might be given to patients with a broader spectrum of performance status. Trials are ongoing with addition of various targeted agents with these two standard chemotherapy backbones. Data for second and third line treatment are emerging. Treatment agents targeting stroma, immune pathways and inflammation are under development.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- Saif MW. Pancreatic neoplasm in 2011: an update. JOP 2011;12:316-21. [PubMed]
- Bilimoria KY, Bentrem DJ, Ko CY, et al. National failure to operate on early stage pancreatic cancer. Ann Surg 2007;246:173-80. [PubMed]
- Mayo SC, Nathan H, Cameron JL, et al. Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer 2012;118:2674-81. [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [PubMed]
- Hidalgo M. Pancreatic cancer. N Engl J Med 2010;362:1605-17. [PubMed]
- Heinemann V, Haas M, Boeck S. Systemic treatment of advanced pancreatic cancer. Cancer Treat Rev 2012;38:843-53. [PubMed]
- Warsame R, Grothey A. Treatment options for advanced pancreatic cancer: a review. Expert Rev Anticancer Ther 2012;12:1327-36. [PubMed]
- Zafar SF, El-Rayes BF. Chemotherapeutic Strategies in Advanced or Metastatic Pancreatic Adenocarcinoma. Am J Clin Oncol 2012. [Epub ahead of print]. [PubMed]
- Catenacci DVT, Bahary N, Edelman MJ, et al. A phase IB/randomized phase II study of gemcitabine (G) plus placebo (P) or vismodegib (V), a hedgehog (Hh) pathway inhibitor, in patients (pts) with metastatic pancreatic cancer (PC): Interim analysis of a University of Chicago phase II consortium study. 2012 ASCO Annual Meeting Abstract No: 4022.
- Deplanque D, Demarchi D, Hebbar M, et al. Masitinib in nonresectable pancreatic cancer: Results of a phase III randomized placebo-controlled trial. J Clin Oncol 2013;31:abstr 158.
- Gonçalves A, Gilabert M, François E, et al. BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann Oncol 2012;23:2799-805. [PubMed]
- Gemcitabine and AMG 479 in Metastatic Adenocarcinoma of the Pancreas. NCT01231347.
- A Study Evaluating IPI-926 in Combination With Gemcitabine in Patients With Metastatic Pancreatic Cancer. NCT01130142.
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [PubMed]
- Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol 2009;27:5513-8. [PubMed]
- Heinemann V, Boeck S, Hinke A, et al. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer 2008;8:82. [PubMed]
- Feig C, Gopinathan A, Neesse A, et al. The pancreas cancer microenvironment. Clin Cancer Res 2012;18:4266-76. [PubMed]
- Neesse A, Michl P, Frese KK, et al. Stromal biology and therapy in pancreatic cancer. Gut 2011;60:861-8. [PubMed]
- Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol 2011;29:4548-54. [PubMed]
- Garrido-Laguna I, Uson M, Rajeshkumar NV, et al. Tumor engraftment in nude mice and enrichment in stroma- related gene pathways predict poor survival and resistance to gemcitabine in patients with pancreatic cancer. Clin Cancer Res 2011;17:5793-800. [PubMed]
- Cubillo A, Calles A, López-Casas PP, et al. Feasibility to obtain a chemogram in circulating tumorigenic cells to guide further treatments in refractory solid tumors. J Clin Oncol 2012;30:abstr 3066.
- Von Hoff DD, Stephenson JJ Jr, Rosen P, et al. Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. J Clin Oncol 2010;28:4877-83. [PubMed]
- A Study of Therapy Selected by Molecular/Metabolic Profiling in Patients With Previously Treated Metastatic Pancreatic Cancer. NCT01196247.
- Sangar V, Ricigliano M, O’Reilly EM et al. Use of pharmacogenomic modeling in pancreatic cancer for prediction of chemotherapy response and resistance. J Clin Oncol 2013;31:abstr 142.
- Gemcitabine and ON 01910.Na in Previously Untreated Metastatic Pancreatic Cancer. NCT01360853.
- Clinical Trial Testing TH-302 in Combination With Gemcitabine in Previously Untreated Subjects With Metastatic or Locally Advanced Unresectable Pancreatic Adenocarcinoma. NCT01746979.
- A Study to See if hENT1 Testing on Tumour Tissue Can Predict Response to Treatment With Gemcitabine Chemotherapy and if a Different Chemotherapy Called FOLFOX is Better Than Gemcitabine in Metastatic Pancreas Cancer. NCT01586611.
- PEGPH20 Plus Nab-Paclitaxel Plus Gemcitabine Compared With Nab-Paclitaxel Plus Gemcitabine in Subjects With Stage IV Untreated Pancreatic Cancer. NCT01839487.
- M402 in Combination With Nab-Paclitaxel and Gemcitabine in Pancreatic Cancer. NCT01621243.
- A Study in Metastatic Cancer and Advanced or Metastatic Unresectable Pancreatic Cancer. NCT01373164.
- Hedgehog Inhibitors for Metastatic Adenocarcinoma of the Pancreas. NCT01088815.
- Gemcitabine Hydrochloride With or Without Vismodegib in Treating Patients With Recurrent or Metastatic Pancreatic Cancer. NCT01064622.
- Vismodegib and Gemcitabine Hydrochloride in Treating Patients With Advanced Pancreatic Cancer. NCT01195415.
- LDE225 With Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan for Untreated Advanced Pancreatic Cancer. NCT01485744.
- A Phase 1b/2 Study of OMP-59R5 in Combination With Nab-Paclitaxel and Gemcitabine in Subjects With Previously Untreated Stage IV Pancreatic Cancer. NCT01647828.
- MK0752 and Gemcitabine Hydrochloride in Treating Patients With Stage III and IV Pancreatic Cancer That Cannot Be Removed by Surgery. NCT01098344
- Phase II Trial Of Gemcitabine Plus Nab-Paclitaxel +/- OGX-427 In Patients With Metastatic Pancreatic Cancer. NCT01844817.
- Trial of Gemcitabine With or Without MSC1936369B in Pancreatic Cancer. NCT01016483.
- A Safety and Efficacy Study of RX-0201 Plus Gemcitabine in Metastatic Pancreatic Cancer. NCT01028495.
- Afatinib as Cancer Therapy for Exocrine Pancreatic Tumours. NCT01728818.
- A Clinical Trial of Anti-Angiogenic Drug Combination Tl-118 for Pancreatic Cancer Patients Who Are Starting Gemcitabine Treatment. NCT01509911.
- A Phase 2 Study of LY2495655 in Participants With Pancreatic Cancer. NCT01505530.
- Carboplatin and Paclitaxel With or Without Viral Therapy in Treating Patients With Recurrent or Metastatic Pancreatic Cancer. NCT01280058.
- Gemcitabine Hydrochloride and Cisplatin With or Without Veliparib or Veliparib Alone in Patients With Locally Advanced or Metastatic Pancreatic Cancer. NCT01585805.
- Metformin Combined With Chemotherapy for Pancreatic Cancer. NCT01210911.
- Treatment of Patients Suffering From a Progressive Pancreas Carcinoma With Everolimus (RAD001) and Gemcitabine. NCT00560963.
- Combination Chemotherapy With or Without Metformin Hydrochloride in Treating Patients With Metastatic Pancreatic Cancer . NCT01167738.
- Vorinostat in Combination With Radiation Therapy and Infusional Fluorouracil (5-FU) in Patients With Locally Advanced Adenocarcinoma of the Pancreas. NCT00948688.
- Phase I Trial of 5-Azacitidine Plus Gemcitabine in Patients With Advanced Pancreatic Cancer. NCT01167816.
- Pelzer U, Schwaner I, Stieler J, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer 2011;47:1676-81. [PubMed]
- Study of MM-398 With or Without 5-Fluorouracil and Leucovorin, Versus 5-Fluorouracil and Leucovorin in Patients With Metastatic Pancreatic Cancer. NCT01494506.
- Study of Ruxolitinib in Pancreatic Cancer Patients. NCT01423604.
- Glufosfamide Versus 5-FU in Second Line Metastatic Pancreatic Cancer. NCT01954992.
- Selumetinib and Akt Inhibitor MK2206 or mFOLFOX Therapy Comprising Oxaliplatin and Fluorouracil in Treating Patients With Metastatic Pancreatic Cancer Previously Treated With Chemotherapy. NCT01658943.
- Selumetinib and Erlotinib Hydrochloride in Treating Patients With Locally Advanced or Metastatic Pancreatic Cancer. NCT01222689.
- Sorafenib Tosylate and Everolimus in Treating Patients With Advanced Solid Tumors and Metastatic Pancreatic Cancer That Does Not Respond to Gemcitabine Hydrochloride. NCT00981162.
- Stepanski EJ, Reyes C, Walker MS, et al. The association of rash severity with overall survival: findings from patients receiving erlotinib for pancreatic cancer in the community setting. Pancreas 2013;42:32-6. [PubMed]
- Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol 2010;28:3605-10. [PubMed]
- Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 2009;27:2231-7. [PubMed]
- Strumberg D, Schultheis B, Ebert MP, et al. Phase II, randomized, double-blind placebo-controlled trial of nimotuzumab plus gemcitabine compared with gemcitabine alone in patients (pts) with advanced pancreatic cancer (PC). J Clin Oncol 2013;31:abstr 4009.
- Kindler HL, Ioka T, Richel DJ, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol 2011;12:256-62. [PubMed]
- Kindler HL, Wroblewski K, Wallace JA, et al. Gemcitabine plus sorafenib in patients with advanced pancreatic cancer: a phase II trial of the University of Chicago Phase II Consortium. Invest New Drugs 2012;30:382-6. [PubMed]
- Van Cutsem E, van de Velde H, Karasek P, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol 2004;22:1430-8. [PubMed]
- Deplanque G, Hebbar M, Flynn PJ, et al. masitinib in nonresectable pancreatic cancer: Results of a phase III randomized placebo-controlled trial. J Clin Oncol 2013; 31:abstr 158.
- Bramhall SR, Schulz J, Nemunaitis J, et al. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer 2002;87:161-7. [PubMed]
- Philip PA, Goldman BH, Ramanathan RK, et al. Phase I randomized phase II trial of gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus erlotinib as first-line treatment in patients with metastatic pancreatic cancer (SWOG-0727). J Clin Oncol 2012;30:abstr 198.
- Infante JR, Somer BG, Park JO, et al. A randomized, double-blind, placebo-controlled trial of trametinib, a MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. J Clin Oncol 2013;31:abstr 291.
- BKM120 + mFOLFOX6 in Advanced Solid Tumors With Expansion Cohort Pancreatic Cancer. NCT01571024.
- Safety, Pharmacokinetics and Pharmacodynamics of BEZ235 Plus MEK162 in Selected Advanced Solid Tumor Patients. NCT01337765.
- Wolpin BM, Hezel AF, Abrams T, et al. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol 2009;27:193-8. [PubMed]
- Erlotinib and RAD001 (Everolimus) in Patients With Previously Treated Advanced Pancreatic Cancer. NCT00640978.
- CCI-779 in Treating Patients With Locally Advanced or Metastatic Pancreatic Cancer. NCT00075647.
- Sorafenib Tosylate and Everolimus in Treating Patients With Advanced Solid Tumors and Metastatic Pancreatic Cancer That Does Not Respond to Gemcitabine Hydrochloride. NCT00981162.
- Chung VM, McDonough S, Philip PA, et al. SWOG S1115: Randomized phase II clinical trial of selumetinib (AZD6244; ARRY 142886) hydrogen sulfate (NSC-748727) and MK-2206 (NSC-749607) versus mFOLFOX in patients withmetastatic pancreatic cancer after prior chemotherapy. J Clin Oncol 2013;31:TPS4145.
- Safety Study of XL765 (SAR245409) in Combination With Erlotinib in Adults With Solid Tumors. NCT00777699.
- Gamma-Secretase Inhibitor RO4929097 and Gemcitabine Hydrochloride in Treating Patients With Advanced Solid Tumors. NCT01145456.
- A Study of AGS-1C4D4 Given in Combination With Gemcitabine in Subjects With Metastatic Pancreatic Cancer. NCT00902291.
- Chee CE, Krishnamurthi S, Nock CJ, et al. Phase II study of dasatinib (BMS-354825) in patients with metastatic adenocarcinoma of the pancreas. Oncologist 2013;18:1091-2. [PubMed]
- Renouf DJ, Moore MJ, Hedley D, et al. A phase I/II study of the Src inhibitor saracatinib (AZD0530) in combination with gemcitabine in advanced pancreatic cancer. Invest New Drugs 2012;30:779-86. [PubMed]
- Study to Assess the Safety & Tolerability of a PARP Inhibitor in Combination With Gemcitabine in Pancreatic Cancer. NCT00515866.
- Le DT, Lutz E, Uram JN, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother 2013;36:382-9. [PubMed]
- A Phase 1/2, Open-label Study of Nivolumab Monotherapy or Nivolumab Combined With Ipilimumab in Subjects With Advanced or Metastatic Solid Tumors. NCT01928394.
- Pollak M. Metformin and pancreatic cancer: a clue requiring investigation. Clin Cancer Res 2012;18:2723-5. [PubMed]
- Neesse A, Michl P, Frese KK, et al. Stromal biology and therapy in pancreatic cancer. Gut 2011;60:861-8. [PubMed]
- Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med 2009;361:1164-72. [PubMed]
- Ristorcelli E, Lombardo D. Targeting Notch signaling in pancreatic cancer. Expert Opin Ther Targets 2010;14:541-52. [PubMed]
- Sjölund J, Manetopoulos C, Stockhausen MT, et al. The Notch pathway in cancer: differentiation gone awry. Eur J Cancer 2005;41:2620-9. [PubMed]
- O'Reilly EM, Smith LS, Bendell JC, et al. Phase Ib of anticancer stem cell antibody OMP-59R5 (anti-Notch2/3) in combination with nab-paclitaxel and gemcitabine (Nab-P+Gem) in patients (pts) with untreated metastatic pancreatic cancer (mPC). J Clin Oncol 2014;32:abstr 220.
- Gamma-Secretase/Notch Signalling Pathway Inhibitor RO4929097 in Treating Patients With Previously Treated Metastatic Pancreatic Cancer. NCT01232829.
- Fuxe J, Karlsson MC. TGF-β-induced epithelial-mesenchymal transition: a link between cancer and inflammation. Semin Cancer Biol 2012;22:455-61. [PubMed]
- Oettle H, Seufferlein T, Luger T, et al. Final results of a phase I/II study in patients with pancreatic cancer, malignant melanoma, and colorectal carcinoma with trabedersen. J Clin Oncol 2012;30:abstr 4034.
- Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21:418-29. [PubMed]
- Thompson CB, Shepard HM, O’Connor PM, et al. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol Cancer Ther 2010;9:3052-64. [PubMed]
- Hingorani SR, Harris WP, Beck JT, et al. A phase Ib study of gemcitabine plus PEGPH20 (pegylated recombinant human hyaluronidase) in patients with stage IV previously untreated pancreatic cancer. J Clin Oncol 2013;31:abstr 4010.
- S1313, Phase IB/II Randomized Study of MFOLFIRINOX + PEGPH20 Vs MFOLFIRINOX Alone in Patients With Good Performance Status Metastatic Pancreatic Adenocarcinoma. NCT01959139.
- Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer 2004;4:814-9. [PubMed]
- Helleday T, Bryant HE, Schultz N. Poly(ADP-ribose) polymerase (PARP-1) in homologous recombination and as a target for cancer therapy. Cell Cycle 2005;4:1176-8. [PubMed]
- Friedenson B. BRCA1 and BRCA2 pathways and the risk of cancers other than breast or ovarian. MedGenMed 2005;7:60. [PubMed]
- Ferrone CR, Tang LH, Tomlinson J, et al. Determining prognosis in patients with pancreatic endocrine neoplasms: can the WHO classification system be simplified? J Clin Oncol 2007;25:5609-15. [PubMed]
- Couch FJ, Johnson MR, Rabe KG, et al. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2007;16:342-6. [PubMed]
- McCabe N, Lord CJ, Tutt AN, et al. BRCA2-deficient CAPAN-1 cells are extremely sensitive to the inhibition of Poly (ADP-Ribose) polymerase: an issue of potency. Cancer Biol Ther 2005;4:934-6. [PubMed]
- Goggins M, Schutte M, Lu J, et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Res 1996;56:5360-4. [PubMed]
- Liu X, Shi Y, Maag DX, et al. Iniparib nonselectively modifies cysteine-containing proteins in tumor cells and is not a bona fide PARP inhibitor. Clin Cancer Res 2012;18:510-23. [PubMed]
- Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res 2008;14:1291-5. [PubMed]
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 2007;7:573-84. [PubMed]
- Byrski T, Gronwald J, Huzarski T, et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol 2010;28:375-9. [PubMed]
- Silver DP, Richardson AL, Eklund AC, et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol 2010;28:1145-53. [PubMed]
- Kimura Y, Tsukada J, Tomoda T, et al. Clinical and immunologic evaluation of dendritic cell-based immunotherapy in combination with gemcitabine and/or S-1 in patients with advanced pancreatic carcinoma. Pancreas 2012;41:195-205. [PubMed]
- Morse MA, Nair SK, Boczkowski D, et al. The feasibility and safety of immunotherapy with dendritic cells loaded with CEA mRNA following neoadjuvant chemoradiotherapy and resection of pancreatic cancer. Int J Gastrointest Cancer 2002;32:1-6. [PubMed]
- Kotera Y, Fontenot JD, Pecher G, et al. Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients. Cancer Res 1994;54:2856-60. [PubMed]
- Ramanathan RK, Lee KM, McKolanis J, et al. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol Immunother 2005;54:254-64. [PubMed]
- Rong Y, Qin X, Jin D, et al. A phase I pilot trial of MUC1-peptide-pulsed dendritic cells in the treatment of advanced pancreatic cancer. Clin Exp Med 2012;12:173-80. [PubMed]
- Kaufman HL, Kim-Schulze S, Manson K, et al. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J Transl Med 2007;5:60. [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6.
- Maki RG, Livingston PO, Lewis JJ, et al. A phase I pilot study of autologous heat shock protein vaccine HSPPC-96 in patients with resected pancreatic adenocarcinoma. Dig Dis Sci 2007;52:1964-72. [PubMed]
- Galili U, Shohet SB, Kobrin E, et al. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem 1988;263:17755-62. [PubMed]
- Rossi GR, Mautino MR, Unfer RC, et al. Effective treatment of preexisting melanoma with whole cell vaccines expressing alpha(1,3)-galactosyl epitopes. Cancer Res 2005;65:10555-61. [PubMed]
- Rossi GR, Hardcare JM, Mulcahy MF, et al. Effect of algenpantucel-L immunotherapy for pancreatic cancer on anti-mesothelin antibody titers and correlation with improved overall survival. J Clin Oncol 2013;31:abstr 3007.
- Lutz E, Yeo CJ, Lillemoe KD, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg 2011;253:328-35. [PubMed]
- Almoguera C, Shibata D, Forrester K, et al. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell 1988;53:549-54. [PubMed]
- Gjertsen MK, Buanes T, Rosseland AR, et al. Intradermal ras peptide vaccination with granulocyte-macrophage colony-stimulating factor as adjuvant: Clinical and immunological responses in patients with pancreatic adenocarcinoma. Int J Cancer 2001;92:441-50. [PubMed]
- Vasef MA, Ross JS, Cohen MB. Telomerase activity in human solid tumors. Diagnostic utility and clinical applications. Am J Clin Pathol 1999;112:S68-75. [PubMed]
- Suehara N, Mizumoto K, Kusumoto M, et al. Telomerase activity detected in pancreatic juice 19 months before a tumor is detected in a patient with pancreatic cancer. Am J Gastroenterol 1998;93:1967-71. [PubMed]
- Bernhardt SL, Gjertsen MK, Trachsel S, et al. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: A dose escalating phase I/II study. Br J Cancer 2006;95:1474-82. [PubMed]
- Buanes T, Maurel J, Liauw W, et al. A randomized phase II study of gemcitabine(G) versus GV1001 in sequential combination witg G in patients with unresectable and metastatic panreas cancer. J Clin Oncol 2009;27:abstr 4601.
- GV1001 and Gemcitabine in Sequential Combination to Gemcitabine Monotherapy in Pancreatic Cancer. NCT00358566.
- Gemcitabine and Capecitabine With or Without Vaccine Therapy in Treating Patients With Locally Advanced or Metastatic Pancreatic Cancer. NCT00425360.
- PANVAC™-VF Vaccine for the Treatment of Metastatic Pancreatic Cancer After Failing a Gemcitabine-Containing Regimen. NCT00088660.
- Immunotherapy Study in Borderline Resectable or Locally Advanced Unresectable Pancreatic Cancer. NCT01836432.
- A Phase 2, Multicenter Study of FOLFIRINOX Followed by Ipilimumab With Allogenic GM-CSF Transfected Pancreatic Tumor Vaccine in the Treatment of Metastatic Pancreatic Cancer. NCT01896869.
- Safety and Efficacy of Combination Listeria/GVAX Immunotherapy in Pancreatic Cancer. NCT01417000.
- Safety and Efficacy of Combination Listeria/GVAX Pancreas Vaccine in the Pancreatic Cancer Setting. NCT02004262.
- A Phase I/II Study With CEA(6D) VRP Vaccine in Patients With Advanced or Metastatic CEA-Expressing Malignancies (CEA(6D)VRP). NCT00529984.
- Cyclophosphamide Plus Vaccine Therapy in Treating Patients With Advanced Cancer. NCT00002475.
- Vaccine Therapy, Chemotherapy, and GM-CSF in Treating Patients With Advanced Pancreatic Cancer. NCT00002773.
- Vaccine Therapy, Interleukin-2, and Sargramostim in Treating Patients With Advanced Tumors. NCT00003125.
- Vaccine Therapy Plus Biological Therapy in Treating Adults With Metastatic Solid Tumors. NCT00019331.
- Vaccine Therapy in Treating Patients With Cancer of the Gastrointestinal Tract. NCT00012246.
- Vaccine Therapy, Cyclophosphamide, and Cetuximab in Treating Patients With Metastatic or Locally Advanced Pancreatic Cancer. NCT00305760.
- Vaccine Therapy in Treating Patients With Resected or Locally Advanced Unresectable Pancreatic Cancer. NCT00008099.
- Study of CAP1-6D in Patients With Locally Advanced or Surgically Resected Pancreatic Adenocarcinoma. NCT00203892.
- Antiangiogenic Peptide Vaccine Therapy With Gemcitabine in Treating Patient With Pancreatic Cancer (Phase1/2). NCT00655785.
- Safety Study of Cancer Stem Cell Vaccine to Treat Pancreatic Cancer. NCT02074046.
- Survivin Peptide Vaccination for Patients With Advanced Melanoma, Pancreatic, Colon and Cervical Cancer. NCT00108875.
- Efficacy and Safety of Endoscopic Ultrasound Guided Fine-needle Injection of Dendritic Cells Vaccination Into Unresectable Pancreatic Cancer. NCT01897636.
- Efficacy and Safety of BC-819 and Gemcitabine in Patients With Locally Advanced Pancreatic Adenocarcinoma. NCT01413087.
- Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011;331:1612-6. [PubMed]
- A Trial of Boost Vaccinations of Pancreatic Tumor Cell Vaccine. NCT01088789.
- Zheng L, Edil B, Nguyen T, et al. Novel tertiary lymphoid aggregates induced in pancreatic adenocarcinoma by an allogeneic GM-CSF secreting pancreatic tumor vaccine as a neoadjuvant treatment. 2010 Gastrointestinal Cancers Symposium Abstract No,157.
- Soares KC, Zheng L, Edil B, et al. Vaccines for pancreatic cancer. Cancer J 2012;18:642-52. [PubMed]
- Le DT, Wang-Gillam A, Picozzi V, et al. A phase 2, randomized trial of GVAX pancreas and CRS-207 immunotherapy versus GVAX alone in patients with metastatic pancreatic adenocarcinoma: Updated results. J Clin Oncol 2014;32:abstr 177.
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122-33. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [PubMed]
- Wolpin BM, O'Reilly EM, Ko YJ, et al. Global, multicenter, randomized, phase II trial of gemcitabine and gemcitabine plus AGS-1C4D4 in patients with previously untreated, metastatic pancreatic cancer. Ann Oncol 2013;24:1792-801. [PubMed]


