Upper gastrointestinal neoplasia in familial adenomatous polyposis: prevalence, endoscopic features and management
Introduction
Familial adenomatous polyposis (FAP) is a complex hereditary syndrome which most conspicuous feature is the development of multiple colorectal polyps and a diverse variety of benign and malignant extracolonic manifestations (ECM). Among these ECM, the coexistence of extracolonic polyps has been known for over 100 years after the description in the stomach by Hauser in 1895 (1) and in the duodenum by Funkenstein in 1904 (2).
In FAP, upper digestive lesions include gastric fundic gland polyps (FGP), antrum adenomas, duodenal or small intestinal adenomas and carcinoma. The duodenum is considered the second most common site of polyps after the colorectum (3,4). In this segment, lifetime risks of adenoma and adenocarcinoma approach 100% and 3–5%, respectively. The cumulative cancer incidence was estimated as 18% at 75 years of age (5). Compared to the general population (in which duodenal cancer is rare), the relative risks of duodenal malignancy and ampullary carcinoma are 331 and 124 times greater, respectively (6).
During the 1990s, some important publications addressed the incidence and risk factors associated with malignant degeneration in the upper digestive tract (7-9). At the same time, attempts to identify a genotype-phenotype relationship have been inconsistent (3). Consequently, the upper gastrointestinal (GI) tract has been considered a segment that deserves regular endoscopic surveillance, although the optimal criteria for either endoscopic examinations or management have not yet been fully determined.
Over the years, upper digestive findings in FAP patients have been more critically evaluated as they are considered an important cause of morbidity and mortality. An active search for jejunal lesions has been rarely performed in these patients; moreover, descriptive reports in our country are scarce (10,11). Therefore, the aim of the present study was to evaluate the prevalence of upper digestive lesions (including the stomach, duodenum and jejunum) in FAP patients who were treated and followed in a tertiary public hospital in the last decades. Moreover, we report the results of endoscopic or surgical resection of advanced lesions, reviewing current recommendations for surveillance and management.
Methods
The study was approved by the Gastroenterology Department Ethics Committee approved the present study (Memo-CAPPesq 049/17).
Charts from 140 FAP patients treated in the Colorectal Unit during the last 58 years (from July 1958 to December 2017) were reviewed, focusing on those who underwent upper digestive endoscopic evaluation. Patients who underwent endoscopic examinations signed an informed consent on the aims and morbidity of the procedure.
There were collected clinical (gender, age, family history of FAP and gastroduodenal cancer), endoscopic (age at examination, lesion number, location and histology) and management data (simple biopsy, endoscopic resection/surgical treatment, evolution while on endoscopic surveillance, complications of endoscopic or surgical therapy). This information was retrieved from retrospective [1958–1998] and prospective (after 1998) collected data, when patients started to undergo prospective upper digestive surveillance.
All patients underwent esophagogastroduodenoscopy (EGD) with front-view and/or side-view endoscopes. Examinations were performed with a Fujinon ED 250XT or Olympus JF-130. A small group of patients were also submitted to balloon enteroscopy with a Fujinon EN 450P5 enteroscope.
The location, number, gross appearance and sizes of the polyps or lesions were retrieved. Histological data were derived from tissue extracted from suspected lesion samples or endoscopic/surgical resected specimens, and duodenal adenomatosis was classified according to Spigelman stages, the most used risk-stratification for duodenal cancer (12). Advanced ampullary or duodenal tumors (lesions greater than 10 mm with villous histology and high-grade dysplasia) were also assessed by endoscopic ultrasound (EUS) prior to endoscopic resection to carefully evaluate the dimensions, chances of polypectomy or need for subsequent surgical resection.
Results
During a period of 58 years, 102 out of 140 FAP patients (72.9%) were evaluated with upper digestive endoscopy. Over time, there was an increase in the percentage of FAP patients submitted to upper digestive endoscopic evaluation. Twenty-four out of an initial group of 50 patients (48.0%) evaluated from 1958 to 1998 underwent EGD, increasing to 40/50 patients (80.0%) [1999–2009] and to 38 (100.0%) out of the last 38 patients.
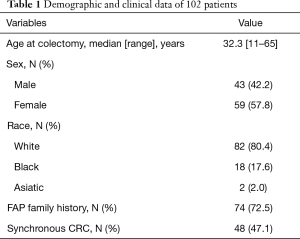
The present series consisted of 59 women (57.8%) and 43 men (42.2%) with a median age of 32.3 years (range, 11–65 years). The majority (80.4%) were recognized as Caucasians (Table 1). A FAP family history and colorectal cancer (CRC) were present in 74 (72.5%) and 48 (47.1%) of the patients, respectively. Most of them presented with endoscopic features of classic FAP.
Full table
During a median follow-up of 74.2 months (range, 4–672 months), all patients underwent 184 (range, 1–8 per patients) endoscopic procedures (1.8 diagnostic or therapeutic endoscopy per patient). Among them, 44 patients (43.1%) underwent more than one upper endoscopic examination. The first endoscopic examination was performed at a median age of 35.9 years (range, 13–75 years), while the last one occurred at 39.5 years (range, 15–77 years).
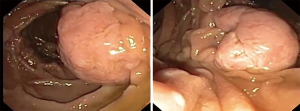
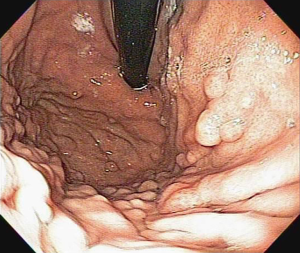
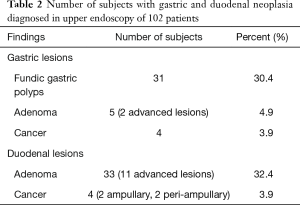
Fundic gastric polyps (Figure 1) were the most common lesions diagnosed with upper endoscopy (Table 2). While 5 adenomas were found in the stomach, 33 patients presented with duodenal or ampullary adenomas (Figures 2,3). Advanced lesions (lesions larger than 10 mm in diameter with high-grade dysplasia, villous or tubule-villous morphology, or any combination of the above features) were detected in the stomach (n=2) and duodenum (n=11).
Full table
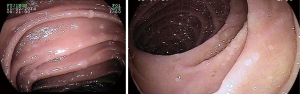
Carcinomas were diagnosed in the stomach (n=4) and duodenum (n=4). Gastric malignancy was diagnosed at 44, 48, 51 and 58 years. Duodenal carcinomas were detected at a mean age of 55.0 years (range, 50–64 years). One patient was diagnosed with stage IV disease at the time of proctocolectomy to resect a CRC and developed an intramucosal carcinoma 1.5 years later. The other three carcinomas were detected during follow-up in patients without previous endoscopy.
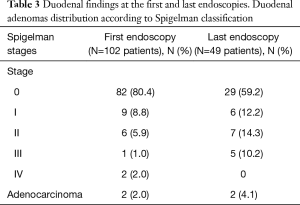
The results regarding duodenal findings are listed in Table 3. Patients at Spigelman 0 stage were predominant at both the first and last endoscopy. Except in stage IV patients, the incidence of more advanced stages progressed over time. The incidence of duodenal carcinoma was greater in the second group. The comparison of duodenal severity between the first and last endoscopies revealed that the Spigelman stage improved in 6 (12.2%) patients, remained unchanged in 25 (51.0%) and worsened in 18 (36.7%).
Full table
A subgroup of 21 patients was also evaluated by enteroscopy. The enteroscope was advanced through median extension of 134 cm (60 to 200 cm) after the Treitz ligament, and jejunal adenomas were found in 12 patients. Within this group, duodenal adenomas were also present in 11 patients.
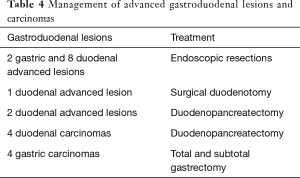
Carcinomas were managed through local resection (endoscopic and surgical), duodenopancreatectomy or gastrectomy (Table 4). Therapeutic complications occurred in two patients; one died after duodenopancreatectomy (due to pulmonary complications), and another patient developed bleeding after endoscopic resection of an advanced lesion.
Full table
Discussion
Since the 1960s, upper digestive screening and surveillance of polyps in FAP has been increasingly advocated (12,13). This idea is based on the almost 100% lifetime risk for duodenal adenomatosis (3,14,15) and the cumulative incidences of Spigelman IV and carcinoma (4% to 10%) within this population (16). Furthermore, duodenal and ampullary malignant tumors are the third most common cause of fatal outcome in FAP patients (17-19).
Traditionally, duodenal polyps vary significantly in their number and size, appearing like plaque-like lesions. Low-risk lesions (small, tubular, and low-grade adenomas) may be managed through standard polypectomy, dissection or local ablation techniques, while surgical options are reserved for more advanced lesions (4,20).
In the present series, duodenal and ampullary adenomas were found in one-third of the patients. These lesions were detected in examinations (first and last endoscopy) performed at median ages varying from 35 to 39 years. Moreover, there were found 13 advanced adenomas (2 gastric and 11 duodenal) and 8 carcinomas (4 gastric and 4 duodenal). Management of these lesions included endoscopic biopsy or resection (mucosal dissection or piecemeal), surgical duodenotomy and gastric or duodenal resections.
Duodenal adenomas tend to appear approximately 15 years after the development of colonic polyps, and their incidence increases over time (21,22). For this reason, duodenal surveillance should begin no later than 25–30 years, which is when the risk of cancer is lower (23,24). Afterwards, endoscopic findings may guide intervals for further evaluations (4). The current recommendations consider that the cancer risk is low (0.7%) among stage 0–III patients and that eventual malignancy development depends on the size, location (ampullary greater than duodenal) and adenomatosis severity (3,14,20).
Therefore, advanced stage patients (size ≥10 mm, villous pattern, and high-grade dysplasia) should undergo endoscopic or transduodenal resection and continued surveillance every 6–12 months (maximum interval of 2 years). They may also receive some sort of chemoprevention (stage III), although duodenal adenomas seem less responsive to chemoprevention with nonsteroidal anti-inflammatory drugs (NSAIDs) than the colonic ones, and there are several side effects/risks associated with their use (4,22). Another option is the indication of prophylactic surgery for stage IV patients (3,8,13).
Although data on ampullary adenomas are scarce, there is a suggestion that ampullary and duodenal diseases should be considered separately (20,25). Ampullary disease deserves an accurate individual risk assessment due to the greater risk of carcinoma compared to nonampullary adenomas, and different surveillance protocols have been proposed with the use of a side-viewing endoscope or chromoendoscopy to improve the detection rates of duodenal polyps (20,26,27).
There is no consensus on the most appropriate surveillance interval following endoscopic resection of ampullary adenomas. As the malignancy risk depends on both ampulla features and staging of the nonampullary duodenal disease, some believe that endoscopic resection may not decrease the need for a more radical operation such as pancreaticoduodenectomy (28,29). Nevertheless, there is a consensus that endoscopic surveillance should be performed after 25 years of age with the use of a side or forward viewer endoscope at intervals defined by staging (annual for stages III and IV, every 3 years for stage II and every 5 years for stage I). There is clear evidence of a benefit in which regular surveillance and cancer prophylactic surgery significantly improved prognosis (5).
When the ampulla and duodenum have no alterations, surveillance every 3–5 years is appropriate, although many patients may have adenomatous pathological changes even if they are macroscopically normal (20,25). The follow-up of 114 patients over 51 months showed no progression in the morphology and histology of the duodenal papilla in 86% and 89% of cases, respectively (25). On the other hand, patients exhibiting major ampullary polyposis (polyp >1 cm with moderate/severe dysplasia or a villous pattern) are more prone to malignancy than those with minor polyposis (less than 1 cm with mild dysplasia and no villous component) (30). Therefore, annual surveillance has been proposed for major polyposis (irrespective of Spigelman staging of the nonampullary disease) and every 3 years for minor ampullary polyposis (20). Further assessment of the ampulla may be provided by EUS and magnetic resonance imaging in those considered candidates for endoscopic or surgical resection.
Endoscopic papillectomy is now established as a valuable therapeutic option for adenomas of the Vaterian papilla. Tis or T1 lesions may be managed by endoscopy or surgical ampullectomy when there is no lymphovascular or intraductal invasion and resection of the growth is considered complete (31,32). However, morbidity after endoscopic papillectomy may occur even in experienced hands. In an interesting review, there were reported complications in 23% (range, 10–58%) and a mortality of 0.4% (range, 0–7%) (33). In this regard, one of our young patients presented with pancreatitis after an endoscopic resection of a 1-cm ampullary adenoma.
Although long-term recurrences have been reported in 50–100% after polypectomy, endoscopic management is safe and useful in the early stages. Furthermore, this approach may defer the need for more radical procedures and eventually reduce cancer risk in the long term (34-36). Severe disease has been associated with the time since FAP diagnosis, age and the Spigelman stage at initial endoscopy (37,38). Although it is well recognized that duodenal polyposis progresses in severity (size and dysplasia), the transformation into carcinoma tends to be slow in the setting of aggressive and constant management of advanced polyps, preventing malignant transformation (12). Therefore, avoiding the progression to stage IV disease is particularly crucial because it is associated with a greater risk (1 in 3 patients) of duodenal cancer (8).
In our series, the evaluation of 50 patients with median ages of 35 and 39 years revealed disease progression during a median follow-up of 74.2 months (range, 4–672 months). We identified that while stages 0–II rates decreased from 95% to 84% between the first and last endoscopies, respectively, stages III and IV rates increased from 3% to 10%. In the same period, the crude incidence of duodenal cancer increased from 2% to 4%. Overall, 18 patients (36.7%) progressed to a worse duodenal pattern. Otherwise, most patients (51%) remained unchanged and the Spigelman stages improved in 12%. Some of these cases resolved due to endoscopic resections.
In the literature, adenomatosis reports have been classified as progressive in about 16–44% of cases (5,25,34,39-42), stable in 34–60% and regressive in 12–26% after an interval of 4–14 years (5,25,39).
Similarly, a slow progression (median 4 years) from one Spigelman stage to another has been demonstrated in other cohorts (16,25,41). In a more detailed study, Heiskanen et al. (43) found a mean interval for the progression from stage 0 to stage I of 5.7 years, from I to II of 4 years, from II to III of 6 years and from III to IV of 11 years. Interestingly, the published results suggest that progression in Spigelman categories over time depends more on the size and number than the histologic changes in the polyps (3). Additionally, the incidences of different stages may vary according to methodological differences regarding the length of follow-up, forward versus sideward viewing, number and biopsy location and other features (16).
In our study, only 8 patients (7.8%) had their last endoscopic examination performed more than 15 years ago. Moreover, patients with insufficient data were excluded from evaluation. Since our main aim was to evaluate the prevalence of upper GI polyps, there was no difference in the backgrounds concerning the quality of endoscopy, diagnostic criteria and/or staging and therapy strategy.
Decision analysis concerning surgical treatment must be individualized. In this discussion, the morbidity and mortality after local (duodenotomy with polypectomy and/or ampullectomy) or more radical procedures (pancreas and pylorus sparing duodenectomy, cephalic pancreaticoduodenectomy) must be weighed against the risk of duodenal malignancy and clinical status. Eventually, resection through surgical duodenotomy may be an option in difficult or potentially dangerous cases. Considering that duodenotomy has a 32–43% recurrence rate (9,35,44) and a similar effect on decreasing Spigelman stages (45), transduodenal resection has been exceptionally recommended (46). We had the opportunity to treat a 38-year-old man with a 3-cm villous adenoma, but he developed recurrence only 18 months after duodenotomy. In a recent review on this topic, Brosens et al. (4) reported that although one may expect high recurrence rates (similar to what happens after endoscopic resection), this approach might eventually postpone surgery.
Given the 7–36% risk of duodenal carcinoma, a cancer prophylactic operation has been advocated for advanced cases of duodenal and ampullary adenomatosis or after failed local resection (endoscopic or surgical) (3). We performed pancreaticoduodenectomy in six patients, including two cases of stage IV disease. Among this group, one patient died after a pulmonary thrombosis 30 days after surgery.
This procedure is associated with morbidity as high as 50%, mortality ranging from 2% to 9% and post-operative recurrence in 25–78%, including advanced neoplasia and cancer (20,45,47-49). Therefore, the Whipple operation or a pylorus preserving pancreaticoduodenectomy (PPPD) has been reserved for more advanced lesions or invasive carcinoma (26,50).
After duodenal adenomas, FGP were the most common lesions detected in the present series (31%). FGP consist of hyperplasia of the fundic gland, and micro cysts are usually found in up to 60% of FAP patients (51). Interestingly, although sporadic FGP are considered non-neoplastic lesions in nature (52), they may display adenomatous features with low- and high-grade dysplasia (40%) in the setting of FAP (45,51,53,54). In the analysis of a group of 66 patients, FGP were found in 43 (65%); 36% were low-grade and 3% were high-grade dysplasia.
These characteristics imply a potential risk of progression to gastric cancer (21,55,56). Therefore, if FGP have unusual appearances and are greater than 1 cm in size, they should be biopsied or resected.
In our series, 5 gastric adenomas (2 advanced lesions) and 4 gastric carcinomas were also detected. This increased risk of gastric adenomas in FAP has been widely recognized. The prevalence in the literature ranges from approximately 6–20% in European and American publications (3,42,57,58). In a recent retrospective review of 97 patients undergoing gastroduodenoscopy at the Mayo Clinic from 2004 to 2013, nine patients (9%) had biopsy-proven gastric adenomas; most (n=5) were located in the antrum, and they had a variable size (3–40 mm) and number (0–20 per patient) (59). In a recent series from the Johns Hopkins University (58), the authors found adenomas in 15/66 (23%) of patients. In addition, four cases with pyloric gland adenomas were diagnosed.
Gastric adenomas are not always easily identifiable by endoscopy due to their difficult distinction from cystic FGP located in the fundus and body of the stomach. Usually confined to the antrum, they should be individualized and removed [though endoscopic submucosal dissection (ESD) or endoscopic mucosal resection (EMR) techniques] under a high degree of suspicion during endoscopic evaluation (11,43).
Gastric carcinomas probably originate from adenomas, although some cases may develop from FGP with high-grade dysplasia (60). Four (3.9%) of our patients were diagnosed with gastric cancer during FAP evolution, but it was not possible to define the precursor lesion in any of them. The median ages at FAP diagnosis and gastric cancer detection were 44.7 (range, 39–50) and 50.2 (range, 44–58) years, respectively. Surgical treatment included 3 total and 1 subtotal gastrectomy, including one 51-year-old patient who underwent resection 11 months before colorectal resection for FAP. The other gastric tumors were diagnosed at 106, 30 and 129 months after proctocolectomy.
In a recent Japanese publication, Shibata et al. (61) reported that the mean ages at the time of colectomy and gastric cancer diagnosis were 39.2 and 58 years, respectively. The mean interval between colectomy and gastrectomy was 19 years in five patients. Three out of five patients had multicentric lesions. According to another Japanese series, the incidence of gastric cancer in FAP patients is much greater than in patients without FAP (62).
There is some evidence that gastric cancer incidence in FAP patients differs between Western and Asian countries, which is commonly attributed to the greater prevalence of gastric cancer in Asia. The reported incidences of gastric cancer in Asian series varied from 2.6% in Japan (63) to 4.2% in Korea (64). Iwama et al. (63) estimated the risk of gastric adenocarcinoma to be increased approximately 3-fold in Asian patients with FAP. Conversely, a 0.6% incidence was found in a group of 1,255 patients from EUA (65). In a report from the Johns Hopkins Registry (6), a comparative incidence between FAP patients and the general population showed an increased relative risk of duodenal adenocarcinoma (relative risk, 330.82) and ampullary adenocarcinoma (relative risk, 123.72), but there was no significant increased risk for gastric or non-duodenal small intestinal cancer. Only 1 out of 4 gastric tumors in our series was recognized as Asiatic, suggesting that there was no influence of this factor on the 3.9% gastric cancer incidence we report here.
Data regarding gastroduodenal neoplasms in FAP are scarce in Brazilian series thus far (10,11). To the best of our knowledge, this is the largest series published in our country. We also evaluated a subgroup of 21 patients with enteroscopy, and we detected jejunal adenomas in 12 of them, 11 of whom also presented with duodenal adenomas. The small bowel mucosa status has been addressed by different methods, such as video capsule endoscopy (CE), magnetic resonance enterography (MRE) and balloon-assisted enteroscopy (66). CE has some limitations, such as inaccuracy of size and anatomic polyp localization, as well as carries the risks of evaluating a patient suspected of bowel stricture. MRE seems to be a reliable technique with the advantages of being noninvasive and radiation-free. In a comparative study (67), it was concluded that both CE and MRE might be used for small bowel screening. While CE may detect small lesions that are eventually missed by MRE, MRE may provide mural, perienteric and extraenteric information.
The correct incidence of adenomas distal to the duodenum has not been adequately evaluated thus far, and there have been variable results depending on the examining methodology. Similar to our finding, jejunal polyps were found in 50% of 16 subjects who were evaluated by push enteroscopy (68). Other studies estimated the rate of jejunal and ileal polyps to vary between 30–75% (14). The finding of these lesions has been associated with a severe duodenal polyp burden, although the clinical relevance of lesions beyond the duodenum appears to be limited (69). For this reason, routine jejunoscopy does not seem to be warranted in most patients with FAP (70).
In light of the present study findings, the high prevalence of gastroduodenal polyps in FAP patients justifies the establishment of a routine surveillance program. FAP patients presented an increased risk for upper GI adenomas and cancer, and although gastric lesions are generally a less important clinical issue, we found a 3.9% gastric cancer incidence. At the same time, approximately 1/3 of duodenal lesions progressed slowly throughout follow-up, culminating in a 3.9% incidence of duodenal cancer. Jejunoscopy may only be relevant in cases of severe duodenal disease.
Once the prognosis for duodenal cancer is poor, an aggressive approach to treat advanced gastric adenomas or severe duodenal disease with the removal of suspicious lesions may prevent the growth of high-risk lesions and maximize patient outcomes. In this regard, a French group (71) recently evaluated the long-term efficiency and risks of 245 therapeutic endoscopies performed in 35 stage IV patients. This cohort had a mean classification score of 9.8 points (range, 9–12 points) at first examination. Patients underwent mucosectomy to control significant lesions and argon ablation to treat adenomas smaller than 5 mm. After a mean follow-up of 9 years (range, 1–19 years), the Spigelman scores decreased 6 points in 95% and no patients developed carcinoma. Moreover, only 15 (6%) adverse events occurred. Regardless of these preliminary results, this conservative approach to treat advanced stages still requires confirmation by other centers with the inclusion of a larger number of patients.
As patients reach the fifth decade of life, special attention should be given to the development of upper GI cancer. Given the substantial risks associated with duodenal surgical resection, patients considered for surgery deserve a critical preoperative evaluation and selection (47).
Fortunately, interest regarding the comprehension of risk factors and definition of management guidelines for duodenal polyposis has increased significantly during the last decades (58). Future efforts should address the development of a more refined staging approach as well as effective strategies for prevention and treatment. In this regard, papilla and duodenal features merit different considerations in an improved staging system. Molecular investigations in this field will probably help categorize the risk groups and indicate prognosis.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Gastroenterology Department Ethics Committee approved the present study (Memo-CAPPesq 049/17).
References
- Hauser G. Ueber Polyposis intestinalis adenomatosa und deren Beziehungen zur Krebsentwicklung. Deutsches Archiv für klinische Medizin 1895;55:429-48.
- Funkenstein O. Über Polyposis intestinalis. Zeitschrift für Klinische Medizinische Berlin 1904;55:236-48.
- Bülow S, Björk J, Christensen IJ, et al. Duodenal adenomatosis in familial adenomatous polyposis. Gut 2004;53:381-6. [Crossref] [PubMed]
- Brosens LA, Keller JJ, Offerhaus GJA, et al. Prevention and management of duodenal polyps in familial adenomatous polyposis. Gut 2005;54:1034-43. [Crossref] [PubMed]
- Bülow S, Christensen IJ, Højen H, et al. Duodenal surveillance improves the prognosis after duodenal cancer in familial adenomatous polyposis. Colorectal Dis 2012;14:947-52. [Crossref] [PubMed]
- Offerhaus GJ, Giardiello FM, Krush AJ, et al. The risk of upper gastrointestinal cancer in familial adenomatous polyposis. Gastroenterology 1992;102:1980-2. [Crossref] [PubMed]
- Spigelman AD. Familial adenomatous polyposis and the upper gastrointestinal tract. Semin Colon Rectal Surg 1995;6:26-8.
- Groves CJ, Saunders BP, Spigelman AD, et al. Duodenal cancer in patients with familial adenomatous polyposis (FAP): results of a 10 year prospective study. Gut 2002;50:636-41. [Crossref] [PubMed]
- de Vos tot Nederveen Cappel WH, Järvinen HJ, Björk J, et al. Worldwide survey among polyposis registries of surgical management of severe duodenal adenomatosis in familial adenomatous polyposis. Br J Surg 2003;90:705-10.
- Leal RF, Ayrizono Mde L, Coy CS, et al. Gastroduodenal polyposis in patients with familiar adenomatous polyposis after rectocolectomy. Arq Gastroenterol 2007;44:133-6. [Crossref] [PubMed]
- Campos FG, Habr-Gama A, Kiss DR, et al. Extracolonic manifestations of familial adenomatous polyposis: incidence and impact on the disease outcome. Arq Gastroenterol 2003;40:92-8. [Crossref] [PubMed]
- Spigelman AD, Williams CB, Talbot IC, et al. Upper gastrointestinal cancer in patients with familial adenomatous polyposis. Lancet 1989;2:783-5. [Crossref] [PubMed]
- Church JM, McGannon E, Hull-Boiner S, et al. Gastroduodenal polyps in patients with familial adenomatous polyposis. Dis Colon Rectum 1992;35:1170-3. [Crossref] [PubMed]
- Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis 2009;4:22-5. [Crossref] [PubMed]
- King JE, Dozois RR, Lindor NM, et al. Care of patients and their families with familial adenomatous polyposis. Mayo Clin Proc 2000;75:57-67. [Crossref] [PubMed]
- Mathus-Vliegen EM, Boparai KS, Dekker E, et al. Progression of duodenal adenomatosis in familial adenomatous polyposis: due to ageing of subjects and advances in technology. Familial Cancer 2011;10:491-9. [Crossref] [PubMed]
- de Campos FG, Perez RO, Imperiale AR, et al. Evaluating causes of death in familial adenomatous polyposis. J Gastrointest Surg 2010;14:1943-9. [Crossref] [PubMed]
- Bertario L, Presciuttini S, Sala P, et al. Causes of death and postsurgical survival in familial adenomatous polyposis: results from the Italian Registry. Italian Registry of Familial Polyposis Writing Committee. Semin Surg Oncol 1994;10:225-34. [Crossref] [PubMed]
- Belchetz LA, Berk T, Bapat BV, et al. Changing causes of mortality in patients with familial adenomatous polyposis. Dis Colon Rectum 1996;39:384-7. [Crossref] [PubMed]
- Latchford AR, Neale KF, Spigelman AD, et al. Features of duodenal cancer in patients with familial adenomatous polyposis. Clin Gastroenterol Hepatol 2009;7:659-63. [Crossref] [PubMed]
- Wallace MH, Phillips RK. Upper gastrointestinal disease in patients with familial adenomatous polyposis. Br J Surg 1998;85:742-50. [Crossref] [PubMed]
- Sarre RG, Frost AG, Jagelman DG, et al. Gastric and duodenal polyps in familial adenomatous polyposis: a prospective study of the nature and prevalence of upper gastrointestinal poly. Gut 1987;28:306-14. [Crossref] [PubMed]
- Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666-89. [Crossref] [PubMed]
- Gallagher MC, Phillips RK, Bulow S. Surveillance and management of upper gastrointestinal disease in Familial Adenomatous Polyposis. Fam Cancer 2006;5:263-73. [Crossref] [PubMed]
- Burke CA, Beck GJ, Church JM, et al. The natural history of untreated duodenal and ampullary adenomas in patients with familial adenomatous polyposis followed in an endoscopic surveillance program. Gastrointest Endosc 1999;49:358-64. [Crossref] [PubMed]
- Cordero-Fernández C, Garzón-Benavides M, Pizarro-Moreno A, et al. Gastroduodenal involvement in patients with familial adenomatous polyposis. Prospective study of the nature and evolution of polyps: evaluation of the treatment and surveillance methods applied. Eur J Gastroenterol Hepatol 2009;21:1161-7. [Crossref] [PubMed]
- Dekker E, Boparai KS, Poley JW, et al. High resolution endoscopy and the additional value of chromoendoscopy in the evaluation of duodenal adenomatosis in patients with familial adenomatous polyposis. Endoscopy 2009;41:666-9. [Crossref] [PubMed]
- Baron TH. Ampullary adenoma. Curr Treat Options Gastroenterol 2008;11:96-102. [Crossref] [PubMed]
- Björk J, Akerbrant H, Iselius L, et al. Periampullary adenomas and adenocarcinomas in familial adenomatous polyposis: cumulative risks and APC gene mutations. Gastroenterology 2001;121:1127-35. [Crossref] [PubMed]
- Kashiwagi H, Spigelman AD, Debinski HS, et al. Surveillance of ampullary adenomas in familial adenomatous polyposis. Lancet 1994;344:1582. [Crossref] [PubMed]
- Mantas D, Charalampoudis P, Nikiteas N. FAP related periampullary adenocarcinoma. Int J Surg Case Rep 2013;4:684-6. [Crossref] [PubMed]
- Chini P, Draganov PV. Diagnosis and management of ampullary adenoma: The expanding role of endoscopy. World J Gastrointest Endosc 2011;3:241-7. [Crossref] [PubMed]
- Han J, Kim MH. Endoscopic papillectomy for adenomas of the major duodenal papilla (with video). Gastrointest Endosc 2006;63:292-301. [Crossref] [PubMed]
- Bertoni G, Sassatelli R, Nigrisoli E, et al. High prevalence of adenomas and microadenomas of the duodenal papilla and periampullary region in patients with familial adenomatous polyposis. Eur J Gastroenterol Hepatol 1996;8:1201-6. [Crossref] [PubMed]
- Soravia C, Berk T, Madlensky L, et al. Genotype-phenotype correlations in attenuated adenomatous polyposis coli. Am J Hum Genet 1998;62:1290-301. [Crossref] [PubMed]
- Morpurgo E, Vitale GC, Galandiuk S, et al. Clinical characteristics of familial adenomatous polyposis and management of duodenal adenomas. J Gastrointest Surg 2004;8:559-64. [Crossref] [PubMed]
- Vasen HF, Moslein G, Alonso A, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 2008;57:704-13. [Crossref] [PubMed]
- Saurin JC, Gutknecht C, Napoleon B, et al. Surveillance of duodenal adenomas in familial adenomatous polyposis reveals high cumulative risk of advanced disease. J Clin Oncol 2004;22:493-8. [Crossref] [PubMed]
- Nugent KP, Spigelman AD, Williams CB, et al. Surveillance of duodenal polyps in familial adenomatous polyposis: progress report. J R Soc Med 1994;87:704-6. [PubMed]
- Noda Y, Watanabe H, Iida M, et al. Histologic follow-up of ampullary adenomas in patients with familial adenomatosis coli. Cancer 1992;70:1847-56. [Crossref] [PubMed]
- Matsumoto T. Natural history of ampullary adenoma in familial adenomatous polyposis: reconfirmation of benign nature during extended surveillance. Am J Gastroenterol 2000;95:1557-62. [Crossref] [PubMed]
- Debinski HS, Trojan J, Nugent KP, et al. Effect of sulindac on small polyps in familial adenomatous polyposis. Lancet 1995;345:855-6. [Crossref] [PubMed]
- Heiskanen I, Kellokumpu I, Jarvinen H. Management of duodenal adenomas in 98 patients with familial adenomatous polyposis. Endoscopy 1999;31:412-6. [Crossref] [PubMed]
- Johnson MD, Mackey R, Brown N, et al. Outcome based on management for duodenal adenomas: sporadic versus familial disease. J Gastrointest Surg 2010;14:229-35. [Crossref] [PubMed]
- Lepistö A, Kiviluoto T, Halttunen J, et al. Surveillance and treatment of duodenal adenomatosis in familial adenomatous polyposis. Endoscopy 2009;41:504-9. [Crossref] [PubMed]
- Dixon E, Vollmer CM Jr, Sahajpal A, et al. Transduodenal resection of peri-ampullary lesions. World J Surg 2005;29:649-52. [Crossref] [PubMed]
- van Heumen BW, Nieuwenhuis MH, van Goor H, et al. Surgical management for advanced duodenal adenomatosis and duodenal cancer in Dutch patients with familial adenomatous polyposis: a nationwide retrospective cohort study. Surgery 2012;151:681-90. [Crossref] [PubMed]
- Skipworth JR, Morkane C, Raptis DA, et al. Pancreaticoduodenectomy for advanced duodenal and ampullary adenomatosis in familial adenomatous polyposis. HPB (Oxford) 2011;13:342-9. [Crossref] [PubMed]
- Mackey R, Walsh RM, Chung R, et al. Pancreas-sparing duodenectomy is effective management for familial adenomatous polyposis. J Gastrointest Surg 2005;9:1088-93. [Crossref] [PubMed]
- Caillié F, Paye F, Desaint B, et al. Severe duodenal involvement in familial adenomatous polyposis treated by pylorus-preserving pancreaticoduodenectomy. Ann Surg Oncol 2012;19:2924-31. [Crossref] [PubMed]
- Bianchi LK, Burke CA, Bennett AE, et al. Fundic gland polyp dysplasia is common in familial adenomatous polyposis. Clin Gastroenterol Hepatol 2008;6:180-5. [Crossref] [PubMed]
- Genta RM, Schuler CM, Robiou CI, et al. No association between gastric fundic gland polyps and gastrointestinal neoplasia in a study of over 100,000 patients. Clin Gastroenterol Hepatol 2009;7:849-54. [Crossref] [PubMed]
- Kashiwagi H, Spigelman AD. Gastroduodenal lesions in familial adenomatous polyposis. Surg Today 2000;30:675-82. [Crossref] [PubMed]
- Arnason T, Liang WY, Alfaro E, et al. Morphology and natural history of familial adenomatous polyposis-associated dysplastic fundic gland polyps. Histopathology 2014;65:353-62. [Crossref] [PubMed]
- Garrean S, Hering J, Saied A, et al. Gastric adenocarcinoma arising from fundic gland polyps in a patient with familial adenomatous polyposis syndrome. Am Surg 2008;74:79-83. [PubMed]
- Wu TT, Kornacki S, Rashid A, et al. Dysplasia and dysregulation of proliferation in foveolar and surface epithelia of fundic gland polyps from patients with familial adenomatous polyposis. Am J Surg Pathol 1998;22:293-8. [Crossref] [PubMed]
- Marcello PW, Asbun HJ, Veidenheimer MC, et al. Gastroduodenal polyps in familial adenomatous polyposis. Surg Endosc 1996;10:418-21. [Crossref] [PubMed]
- Wood LD, Salaria SN, Cruise MW, et al. Upper GI tract lesions in familial adenomatous polyposis (FAP): enrichment of pyloric gland adenomas and other gastric and duodenal neoplasms. Am J Surg Pathol 2014;38:389-93. [Crossref] [PubMed]
- Ngamruengphong S, Boardman LA, Heigh RI, et al. Gastric adenomas in familial adenomatous polyposis are common, but subtle, and have a benign course. Hered Cancer Clin Pract 2014;12:4. [Crossref] [PubMed]
- Burt RW. Gastric fundic gland polyps. Gastroenterology 2003;125:1462-9. [Crossref] [PubMed]
- Shibata C, Ogawa H, Miura K, et al. Clinical characteristics of gastric cancer in patients with familial adenomatous polyposis. Tohoku J Exp Med 2013;229:143-6. [Crossref] [PubMed]
- Kamoi R, Iida M, Inoue S, et al. Familial adenomatous polyposis associated with early gastric cancer: report of a case Stomach and Intestine (Tokyo) 1997;32:631-41. (in Japanese).
- Iwama T, Mishima Y, Utsunomiya J. The impact of familial adenomatous polyposis on the tumorigenesis and mortality at the several organs. Its rational treatment. Ann Surg 1993;217:101-8. [Crossref] [PubMed]
- Park SY, Ryu JK, Park JH, et al. Prevalence of gastric and duodenal polyps and risk factors for duodenal neoplasm in korean patients with familial adenomatous polyposis. Gut Liver 2011;5:46-51. [Crossref] [PubMed]
- Jagelman DG, DeCosse JJ, Bussey HJ. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet 1988;1:1149-51. [Crossref] [PubMed]
- Koornstra JJ. Small bowel endoscopy in familial adenomatous polyposis and Lynch syndrome. Best Pract Res Clin Gastroenterol 2012;26:359-68. [Crossref] [PubMed]
- Akin E, Demirezer Bolat A, Buyukasik S, et al. Comparison between Capsule Endoscopy and Magnetic Resonance Enterography for the Detection of Polyps of the Small Intestine in Patients with Familial Adenomatous Polyposis. Gastroenterol Res Pract 2012;2012:215028. [Crossref] [PubMed]
- Bertoni G, Sassatelli R, Tansini P, et al. Jejunal polyps in familial adenomatous polyposis assessed by push-type endoscopy. J Clin Gastroenterol 1993;17:343-7. [Crossref] [PubMed]
- Matsumoto T, Esaki M, Yanaru-Fujisawa R, et al. Small-intestinal involvement in familial adenomatous polyposis: evaluation by double-balloon endoscopy and intraoperative enteroscopy. Gastrointest Endosc 2008;68:911-9. [Crossref] [PubMed]
- Alderlieste YA, Rauws EA, Mathus-Vliegen EM, et al. Prospective enteroscopic evaluation of jejunal polyposis in patients with familial adenomatous polyposis and advanced duodenal polyposis. Fam Cancer 2013;12:51-6. [Crossref] [PubMed]
- Moussata D, Napoleon B, Lepilliez V, et al. Endoscopic treatment of severe duodenal polyposis as an alternative to surgery for patients with familial adenomatous polyposis. Gastrointest Endosc 2014;80:817-25. [Crossref] [PubMed]