Optimal radiation dosing in concurrent neoadjuvant chemoradiation for resectable esophageal cancer: a meta-analysis
Introduction
Esophageal cancer (EC) is the eighth most prevalent cancer worldwide, and the sixth leading cause of cancer death (1). Historically, patients diagnosed with EC were treated with surgery alone but survival outcomes rarely exceeded 20% (2). In an attempt to improve outcomes, researchers in the 1980s began introducing chemotherapy, radiation, or both in addition to resection for locally advanced EC. Adjuvant chemotherapy (3), radiotherapy (4), and chemoradiotherapy (5) strategies were abandoned after poor trial outcomes. Most recent trials have focused on neoadjuvant treatment intensification, with studies comparing neoadjuvant chemotherapy (nCT) and surgery (6), neoadjuvant chemoradiotherapy (nCRT) and surgery (6-21), nCRT and nCT (6,18,22,23), and even nCRT and definitive chemoradiotherapy (24).
The recently updated CROSS trial data (20) provides the strongest evidence for cnCRT over surgery as well as the best outcomes of any treatment for locally advanced EC in a large randomized study. In this trial, patients received neoadjuvant carboplatin and paclitaxel with concurrent radiotherapy (41.4 Gy in 23 fractions) or surgery alone. Median overall survival (OS) was 48.6 months in the nCRT arm compared to 24 months with surgery alone. Several meta-analysis have confirmed improved survival with nCRT compared to surgery (25-30). Greer et al. (31) was the only negative meta-analysis, but even this study showed a trend for increased survival in patients who received nCRT. Five meta-analyses (25,27-29,32) showed increased survival in nCRT compared to nCT. Therefore, nCRT has become the standard of care as evidenced by its utilization in current trials, including both arms of the PROTECT-1402 trial (33) and on one arm of the Neo-AEGIS trial (34).
Nearly all published meta-analyses (25-29,31,32) contained heterogeneous studies with nCRT that included induction chemotherapy (22,23,35) and sequential chemoradiotherapy (6,8,10). Liu et al. (30) was the sole study to include only concurrent nCRT (cnCRT) trials, albeit including retrospective data. However, the optimal radiation dose which should be used in the setting of cnCRT has not been clearly established. Prescribed doses have ranged from 30 Gy (22) to as high as 50.4 Gy (14). To our knowledge this is the first meta-analysis evaluating outcomes by radiation dose in the setting of prospective RCT using cnCRT for resectable EC. The primary outcome of our study was OS, and the secondary outcome was treatment-related mortality (TRM). We hypothesized that LDRT would have similar OS and TRM to HDRT.
Methods
In this meta-analysis, we sought to compare survival in patients with resectable EC based on radiation dose received as part of a cnCRT protocol, we first conducted a systematic review of current literature and selected studies to be included in our analysis based on a set of eligibility criteria. To be eligible, reviewed studies had to be randomized controlled trials (RCT) which compared cnCRT followed by surgery vs. surgery alone in the initial treatment of esophageal or gastro-esophageal junction carcinomas. Studies were only included if the protocol offered cnCRT in one treatment arm; trials which utilized a sequential chemotherapy and radiotherapy or induction chemotherapy were excluded. Only full-text articles published in English were included in the final analysis. Studies were not excluded on the basis of histology (squamous cell vs. adenocarcinoma) or chemotherapy agents used. Studies were excluded if gastro-esophageal junction (GEJ) was the only sub-site treated in the trial. If the study combined cancers of the GEJ along with cancers of the esophagus proper, then they were included in our analysis.
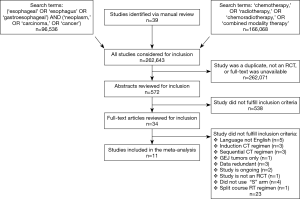
For our initial literature search, we utilized three databases: Medline, Embase, and the Cochrane clinical trials database. The searches were conducted independently by two members of the research team on August 07, 2017. Studies since 1994 were included. Search strategy began with two broad searches. The first search, using the search terms “(‘esophageal’ OR ‘esophagus’ OR ‘gastroesophageal’) AND (‘neoplasm,’ OR ‘carcinoma,’ OR ‘cancer’)” yielded 96,536 citations. The second search, using the terms “‘chemotherapy,’ OR ‘radiotherapy,’ OR ‘chemoradiotherapy,’ OR ‘combined modality therapy’” yielded 166,068 citations. In addition, members of the team manually reviewed reference lists of other meta-analyses; this identified 39 potential studies which were then entered into the final review process.
The citations from the online searches and the manual review were combined to form a common pool of 262,643 citations. These results were subsequently refined to exclude any citation that was redundant, not a RCT or not a full-length article. Refinement yielded 572 citations, which underwent subsequent abstract and full-text screening. During this review process, articles were individually selected for inclusion or exclusion based on the criteria described above; eleven studies were included in the final analysis. The literature review process is summarized in the PRISMA flow diagram in Figure 1.
Articles were screened using Covidence, a web-based platform made for improving healthcare evidence synthesis. Two authors performed both an abstract screening and a subsequent full-text screening to evaluate studies for inclusion into our meta-analysis. Any disagreements regarding abstract or full-text screens were settled by a senior researcher on the team. Study quality was assessed via the CONSORT checklist. We captured data points including follow-up time, radiation dose, OS, stage, performance score (PS), sex, and TRM. Age and comorbidities were not included due to heterogeneity in the reporting.
Radiation doses were converted to biologically effective dose (BED) to make comparisons across fractionation schemes. Forty-eight point eighty-five Gy BED was selected as a cutoff as this is the BED of 41.4 Gy in 23 fractions as delivered in the CROSS trial (20). BED >48.85 Gy was considered high dose radiotherapy (HDRT) and BED ≤48.85 Gy was low dose radiotherapy (LDRT).
Statistics
We used reported hazard ratios (HRs) and corresponding 95% confidence intervals (95% CI) for comparing OS between cnCRT and surgery alone. Standard error (SE) of HR was calculated from 95% CI. When these two quantities were not reported, we calculated HR and corresponding SE based on either reported Kaplan-Meier plot or using the methods of Parmar and Tierney. TRM was analyzed by the risk ratio (RR) and corresponding 95% CI. For the meta-analysis, we used random-effects models based on the DerSimonian and Laird method. Study heterogeneity due to study characteristics between studies was examined by using meta-regression analysis. We conducted meta-regression with publication year, the percentage of female, the percentage of stage III or higher, the percentage of performance status 1, radiation dose, and median follow-up. Statistical heterogeneity across studies was quantified using the Cochran Q statistic and I2 statistic. A pre-planned subgroup analyses was performed for OS excluding the CROSS trial (20) to account for heterogeneity in chemotherapy. All P values of <0.05 (two-tailed) were considered statistically significant. Consort scores for dose groups were compared using Mann-Whitney test. We selected studies which scores were equal to or higher than median (27) and compared scores by dose groups using Mann-Whitney test as well. All statistical analyses were performed using Stata 13 (StataCorp LP, College Station, TX, USA) and RStudio (RStudio, Inc. Boston, MA, USA).
Results
Eleven studies met our inclusion criteria and were entered in the final analysis. Our analysis included one study (15) which did not populate in our initial database search but was identified via manual reference review. One study identified via database search was part of the author’s thesis statement (12), but the full text was not available. Attempts were made to contact the author; however, no response was received. Therefore, the study was not included in the final analysis.
Studies that passed abstract review but not full-text review were not included for the following reasons. Five of the studies were published in a language other than English (36-40); three studies, utilized induction chemotherapy (22,23,35); three studies utilized sequential instead of concurrent CRT (6,8,10); one study looked solely at carcinomas of the GEJ (41); three of the studies featured redundant data (21,42,43); two of the studies remained ongoing (33,34); one of the studies was not an RCT (44); four of the studies did not utilize a “surgery alone” arm (45-48); one study utilized a split course, sequential radiation protocol and did not compare with a “surgery alone” arm (24).
The eleven included studies contained a total of 1,697 patients, 848 randomized to cnCRT and 849 to surgery alone. Of the 848 patients randomized into the cnCRT group, 287 received HDRT and 561 received LDRT. Further details, including radiotherapy and chemotherapy schedules pertaining to each individual study, can be found in Table 1.

Full table
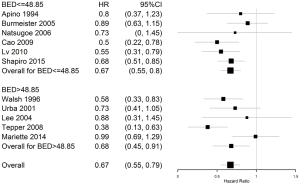
OS was not statistically different between LDRT (HR 0.67; 95% CI, 0.55–0.8) and HDRT (HR 0.68; 95% CI, 0.45–0.91). OS was improved with cnCRT compared to surgery alone (HR 0.67; 95% CI, 0.55–0.79). There was no significant heterogeneity among studies (P=0.10, Q=16, I2=42.4%). The forest plot for HR of death may be seen in Figure 2. A meta-regression was performed to assess whether certain variables may have affected the OS outcomes. All variables studied were non-significant, including median follow-up (P=0.8206), stage III or IV (P=0.5284), female sex (P=0.5968), performance status 1 (P=0.7165), radiation dose in BED (P=0.4840), total sample size (P=0.7434), or year of publication (P=0.9979).
OS outcomes were re-analyzed excluding the CROSS (20) trial to minimize chemotherapy heterogeneity. Still, there was no significant difference between LDRT (HR 0.67; 95% CI, 0.49–0.86) and HDRT (HR 0.68; 95% CI, 0.45–0.91).
TRM was not statistically different between cnCRT and surgery alone (RR 2.97; 95% CI, 0.83–10.64). There was no significant heterogeneity (P=0.90, Q=4.87, I2=0%) among studies. In the cnCRT group, there was no statistical difference in TRM between LDRT (RR 1.2; 95% CI, 0.66–2.16) and HDRT (HR 1.77; 95% CI, 0.83–3.77).
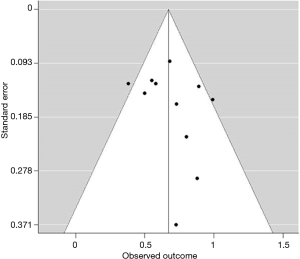
For both OS and TRM outcomes, there was no obvious publication bias among studies included in our analysis. The funnel plot for cnCRT vs. surgery alone showed a symmetrical distribution pattern, as seen in Figure 3.
The overall study quality was assessed by the CONSORT checklist. This showed that most of the studies were of relatively good quality with a median score of 27 and a range of 14–34. There was no statistical difference between the CONSORT scores of LDRT and HDRT (P=0.3142). Stratified analysis of higher quality studies demonstrated the same trend seen in the overall analysis denoting that lesser quality studies did not skew the overall analysis results (P=0.1002).
Discussion
The results of this meta-analysis showed that LDRT (≤48.85 BED) has similar OS and TRM outcomes as HDRT (>48.85 BED) when used as cnCRT in resectable EC.
Multiple previous meta-analyses demonstrated a survival benefit of nCRT when compared to surgery alone (25-30). Only one meta-analysis showed no statistically significant survival advantage for nCRT, but there was a trend towards increased survival compared to surgery (31). Our results fall in line with published literature and showed a statistically significant decreased hazard for death with cnCRT.
The CROSS trial (20) provided the most robust data regarding cnCRT in the treatment of locally advanced EC. This study accrued a large number of patients, 366, along with a long-term follow-up time of 84.4 months. Patients with T2-3N0-1M0 EC received neoadjuvant carboplatin and placlitaxel for five cycles with concurrent radiotherapy of 41.4 Gy in 23 fractions before surgery or surgery alone. Compared to surgery alone, cnCRT increased R0 resections from 69% to 92% (P<0.001), decreased positive pathological nodes from 75% to 31% (P<0.001), and resulted in a complete pathologic response (pCR) rate of 29% (42). Median OS was 48.6 months in the nCRT arm compared to 24 months with S (20). Only Natsugoe et al. showed a superior 5-year OS with cnCRT, but this trial only included 45 patients with only 24 months of follow-up (17).
Two ongoing clinical trials investigating optimal treatment for locally advanced EC include cnCRT (33,34). PROTECT-1402 is a phase II study that compares cnCRT including oxaliplatin and fluorouracil (FOLFOX) with carboplatin and fluorouracil (5-FU) in EC; patients in both arms will receive 41.4 Gy in 23 fractions concurrently (33). Neo-AEGIS (34) is a phase III trial which compares CROSS trial cnCRT with neoadjuvant etoposide, cisplatin, and 5-FU, which was found to improve survival compared to surgery alone in gastro-EC (49). Given the implementation of cnCRT in the design of current clinical trials (33,34), in addition to the results of the CROSS trial (20), cnCRT has established itself as the current standard of care in the treatment of EC.
One of the strengths of our study is that it only includes prospective, pure cnCRT trials. The majority of previous meta-analyses (25-29,31) contained heterogeneous studies with nCRT that included induction chemotherapy (22,23,35) and sequential chemoradiotherapy (6,8,10). Liu et al. (30) is the only other meta-analysis to our knowledge that included only cnCRT. It too showed a survival advantage of cnCRT compared to surgery.
While ongoing RCT are trying to establish the ideal chemotherapy regimen for cnCRT, one of the major questions which remain unanswered regarding cnCRT is the appropriate radiation dose. NCCN guidelines advocate for 41.4 to 50.4 Gy, stating that patients who are at risk of not undergoing surgery should receive higher doses as a lower dose would not be adequate for definitive treatment (50). With varying radiation doses in the prospective trials, previously published meta-analyses did not address this question. Our study is the first to look at survival outcomes by radiation dose, aiming to define whether a CROSS trial radiation dose of 48.85 BED is sufficient. We found that both OS and TRM were not statistically different between cnCRT with LDRT and HDRT.
OS outcomes are affected by the balance between oncologic response rates and toxicity. Determining the appropriate dose for the optimal therapeutic gain has been difficult due to inconsistency in the literature. Ordu et al. found that increasing neoadjuvant radiotherapy dose results in higher rates of pCR but also in higher grade 3 or 4 non-hematologic acute toxicity (51). However, a recent National Cancer Data Base study found that OS, 30-day re-admission, 30-day mortality, or length of postoperative hospital stay did not vary with radiotherapy dose (52). This is in line with the results of our meta-analysis.
The limitations of this study include small sample sizes as well as heterogeneity of tumor types, staging techniques, surgical techniques, radiation doses, and chemotherapy regimens. The meta-regression demonstrated that there were no significant differences between the groups that were predictive of OS including median follow-up, advanced stage disease, sex, performance status, radiation dose in BED, sample size, and year of publication.
One might hypothesize that the LDRT group’s survival outcomes were improved by the CROSS trial (20), whose OS results exceed the rest. This is the only study to use a chemotherapy regimen of carboplatin and paclitaxel; the remainder of the studies in our analysis utilized cisplatin-based chemotherapy. We therefore excluded the CROSS trial (20) in a pre-planned analysis by dose to minimize chemotherapy heterogeneity. On this analysis including only cisplatin-based chemotherapies, there was still no difference in OS between LDRT and HDRT. Therefore, 48.85 Gy BED may be adequate in the cnCRT setting regardless of chemotherapy used.
Conclusions
In conclusion, this meta-analysis shows that 48.85 Gy BED, or 41.4 Gy in 23 fractions, may be an adequate dose for cnCRT treatment of resectable EC; this dose has equivalent OS and TRM to HDRT (>48.85 Gy BED). We suggest creating treatment plans to 50.4 Gy with an intent to deliver 41.4 Gy. If at that point, the performance score is adequate, then the patient should proceed to surgery without completing the prescription to 50.4 Gy. If clinical or radiographic evaluation indicates that the patient is not fit for surgery, then chemoradiation should continue to 50.4 Gy to deliver a definitive dose.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Ando N, Ozawa S, Kitagawa Y, et al. Improvement in the results of surgical treatment of advanced squamous esophageal carcinoma during 15 consecutive years. Ann Surg 2000;232:225-32. [Crossref] [PubMed]
- Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study--JCOG9204. J Clin Oncol 2003;21:4592-6. [Crossref] [PubMed]
- Zieren HU, Muller JM, Jacobi CA, et al. Adjuvant postoperative radiation therapy after curative resection of squamous cell carcinoma of the thoracic esophagus: a prospective randomized study. World J Surg 1995;19:444-9. [Crossref] [PubMed]
- Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol 2007;8:545-53. [Crossref] [PubMed]
- Nygaard K, Hagen S, Hansen HS, et al. Pre-operative radiotherapy prolongs survival in operable esophageal carcinoma: a randomized, multicenter study of pre-operative radiotherapy and chemotherapy. The second Scandinavian trial in esophageal cancer. World J Surg 1992;16:1104-9; discussion 1110. [Crossref] [PubMed]
- Apinop C, Puttisak P, Preecha N. A prospective study of combined therapy in esophageal cancer. Hepatogastroenterology 1994;41:391-3. [PubMed]
- Le Prise E, Etienne PL, Meunier B, et al. A randomized study of chemotherapy, radiation therapy, and surgery versus surgery for localized squamous cell carcinoma of the esophagus. Cancer 1994;73:1779-84. [Crossref] [PubMed]
- Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305-13. [Crossref] [PubMed]
- Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 1997;337:161-7. [Crossref] [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [Crossref] [PubMed]
- Walsh T. The role of multimodality therapy in improving survival: a prospective randomised trial. In: Predicting, defining and improving outcomes for oesophageal carcinoma [MD thesis]. Dublin: Trinity College, University of Dublin, 1995:124-50.
- Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005;6:659-68. [Crossref] [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [Crossref] [PubMed]
- Lv J, Cao XF, Zhu B, et al. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J Gastroenterol 2010;16:1649-54. [Crossref] [PubMed]
- Lee JL, Park SI, Kim SB, et al. A single institutional phase III trial of preoperative chemotherapy with hyperfractionation radiotherapy plus surgery versus surgery alone for resectable esophageal squamous cell carcinoma. Ann Oncol 2004;15:947-54. [Crossref] [PubMed]
- Natsugoe S, Okumura H, Matsumoto M, et al. Randomized controlled study on preoperative chemoradiotherapy followed by surgery versus surgery alone for esophageal squamous cell cancer in a single institution. Dis Esophagus 2006;19:468-72. [Crossref] [PubMed]
- Cao XF, He XT, Ji L, et al. Effects of neoadjuvant radiochemotherapy on pathological staging and prognosis for locally advanced esophageal squamous cell carcinoma. Dis Esophagus 2009;22:477-81. [Crossref] [PubMed]
- Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014;32:2416-22. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Walsh TN, Grennell M, Mansoor S, et al. Neoadjuvant treatment of advanced stage esophageal adenocarcinoma increases survival. Dis Esophagus 2002;15:121-4. [Crossref] [PubMed]
- Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851-6. [Crossref] [PubMed]
- Burmeister BH, Thomas JM, Burmeister EA, et al. Is concurrent radiation therapy required in patients receiving preoperative chemotherapy for adenocarcinoma of the oesophagus? A randomised phase II trial. Eur J Cancer 2011;47:354-60. [Crossref] [PubMed]
- Bedenne L, Michel P, Bouche O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160-8. [Crossref] [PubMed]
- Kaklamanos IG, Walker GR, Ferry K, et al. Neoadjuvant treatment for resectable cancer of the esophagus and the gastroesophageal junction: a meta-analysis of randomized clinical trials. Ann Surg Oncol 2003;10:754-61. [Crossref] [PubMed]
- Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226-34. [Crossref] [PubMed]
- Graham AJ, Shrive FM, Ghali WA, et al. Defining the optimal treatment of locally advanced esophageal cancer: a systematic review and decision analysis. Ann Thorac Surg 2007;83:1257-64. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Pasquali S, Yim G, Vohra RS, et al. Survival After Neoadjuvant and Adjuvant Treatments Compared to Surgery Alone for Resectable Esophageal Carcinoma: A Network Meta-analysis. Ann Surg 2017;265:481-91. [Crossref] [PubMed]
- Liu B, Bo Y, Wang K, et al. Concurrent neoadjuvant chemoradiotherapy could improve survival outcomes for patients with esophageal cancer: a meta-analysis based on random clinical trials. Oncotarget 2017;8:20410-7. [PubMed]
- Greer SE, Goodney PP, Sutton JE, et al. Neoadjuvant chemoradiotherapy for esophageal carcinoma: a meta-analysis. Surgery 2005;137:172-7. [Crossref] [PubMed]
- Fan M, Lin Y, Pan J, et al. Survival after neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for resectable esophageal carcinoma: A meta-analysis. Thorac Cancer 2016;7:173-81. [Crossref] [PubMed]
- Messager M, Mirabel X, Tresch E, et al. Preoperative chemoradiation with paclitaxel-carboplatin or with fluorouracil-oxaliplatin-folinic acid (FOLFOX) for resectable esophageal and junctional cancer: the PROTECT-1402, randomized phase 2 trial. BMC Cancer 2016;16:318. [Crossref] [PubMed]
- Reynolds JV, Preston SR, O'Neill B, et al. ICORG 10-14: NEOadjuvant trial in Adenocarcinoma of the oEsophagus and oesophagoGastric junction International Study (Neo-AEGIS). BMC Cancer 2017;17:401. [Crossref] [PubMed]
- Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310-7. [Crossref] [PubMed]
- An FS, Huang JQ, Xie YT, et al. A prospective study of combined chemoradiotherapy followed by surgery in the treatment of esophageal carcinoma. Zhonghua Zhong Liu Za Zhi 2003;25:376-9. [PubMed]
- Yang H, Fu JH, Liu MZ, et al. A multi-centered randomized controlled study of neo-adjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of esophagus: an interim analysis. Zhonghua Yi Xue Za Zhi 2012;92:1028-32. [PubMed]
- Peng L, Xie TP, Han YT, et al. Randomized controlled study on preoperative concurrent chemoradiotherapy versus surgery alone for esophageal squamous cell carcinoma. Tumor 2008;28:620-2.
- Jin FL, Hu ZL, Ma HF. Treatment effect of neoadjuvant chemoradiotherapy followed by surgery versus surgery alone in local advanced esophageal carcinoma. J Pract Oncol 2011;26:523-6.
- Cao XF, Lu J, Zhu B, et al. A prospective comparison between surgery alone and postoperative chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. Zhonghua Zhong Liu Za Zhi 2010;32:452-5. [PubMed]
- Zhao Q, Li Y, Wang J, et al. Concurrent Neoadjuvant Chemoradiotherapy for Siewert II and III Adenocarcinoma at Gastroesophageal Junction. Am J Med Sci 2015;349:472-6. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- van Heijl M, van Lanschot JJ, Koppert LB, et al. Neoadjuvant chemoradiation followed by surgery versus surgery alone for patients with adenocarcinoma or squamous cell carcinoma of the esophagus (CROSS). BMC Surg 2008;8:21. [Crossref] [PubMed]
- Okumura H, Uchikado Y, Omoto I, et al. The usefulness of neoadjuvant chemoradiation therapy for locally advanced esophageal cancer with multiple lymph-node metastases. Ann Surg Oncol 2014;21:2845-9. [Crossref] [PubMed]
- Tang H, Tan L, Shen Y, et al. CMISG1701: a multicenter prospective randomized phase III clinical trial comparing neoadjuvant chemoradiotherapy to neoadjuvant chemotherapy followed by minimally invasive esophagectomy in patients with locally advanced resectable esophageal squamous cell carcinoma (cT3-4aN0-1M0) (NCT03001596). BMC Cancer 2017;17:450. [Crossref] [PubMed]
- Klevebro F, Johnsen G, Johnson E, et al. Morbidity and mortality after surgery for cancer of the oesophagus and gastro-oesophageal junction: A randomized clinical trial of neoadjuvant chemotherapy vs. neoadjuvant chemoradiation. Eur J Surg Oncol 2015;41:920-6. [Crossref] [PubMed]
- Klevebro F, Alexandersson von Dobeln G, Wang N, et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol 2016;27:660-7. [Crossref] [PubMed]
- Hainsworth JD, Meluch AA, Gray JR, et al. Concurrent chemoradiation followed by esophageal resection vs chemoradiation alone for localized esophageal cancer. Community Oncology 2007;4:431-9. [Crossref]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Esophageal and Esophagogastric Junction Cancers. 2017.
- Ordu AD, Nieder C, Geinitz H, et al. Association between radiation dose and pathological complete response after preoperative radiochemotherapy in esophageal squamous cell cancer. Anticancer Res 2014;34:7255-61. [PubMed]
- Haque W, Verma V, Butler EB, et al. Radiation dose in neoadjuvant chemoradiation therapy for esophageal cancer: patterns of care and outcomes from the National Cancer Data Base. J Gastrointest Oncol 2018;9:80-9. [Crossref] [PubMed]