
Cardiovascular complications of metastatic colorectal cancer treatment
Colorectal cancer (CRC) burden
CRC is one of the most prevailing malignancies, being the third most commonly occurring cancer in men, and the second in women (1,2), making up about 10% of all cancer cases (3). It is the fourth highest cause of cancer death (1). Its incidence increases with increasing age (4). It is more prevalent in developed countries, where more than 65% of cases are found (3). The global burden of CRC is expected to increase by 60% to more than 2.2 million new cases and 1.1 million cancer deaths by 2030 (1).
Approximately 95% of CRC cases concern adenocarcinomas, while the rest include mucinous carcinomas and adenosquamous carcinomas (1). The relative survival rate for CRC is 65% at 5 years following diagnosis and 58% at 10 years (5). Survival rates decrease greatly according to the stage of the disease at the time of diagnosis. Only 39% of CRC patients are diagnosed with localized-stage disease, for which the 5-year survival rate is 90%, while it declines to 14% for metastatic disease (mCRC) (6).
Approximately 50% of CRC patients will develop metastatic disease (7). The most common sites for metastasis for CRC are the liver and the thorax, followed by the peritoneum, the bones and the nervous system (8). The timing of metastases development (synchronous vs. metachronous at the time of diagnosis), their localization (e.g., peritoneal or distant lymph node metastases) and the number of metastatic sites affect crucially the prognosis (9).
The therapeutic approach for mCRC is based on the use of combination cytotoxic therapy that has led to a critical improvement of survival from approximately 1 year during the era of fluoropyrimidine monotherapy to more than 30 months with the integration of multiple cytotoxic agents and targeted therapies (10).
The factors that determine the optimal therapeutic strategy for each mCRC patient are the general condition and performance status, the resectability or not of metastases and the mutational status in terms of BRAF and RAS (11,12). First line therapy in fit patients includes surgery probably with peri-or post-operative chemotherapy, including fluopyrimidines, irinotecan, oxaliplatin, monoclonal antibodies, regorafenib and trifluridine/tipiracil. Alternatively, radiation and local ablation techniques can be used according to the sites of metastases (11,12).
During the first treatment of mCRC, 90% of patients experience more than 1 adverse event (AE) and 39% of them are cardiovascular (CV), followed by AEs from the central nervous system, endocrine/metabolic, respiratory and finally hematologic (13).
CV complications of metastatic CRC treatment
CV complications of mCRC treatment may develop during or after surgery and mostly during chemotherapy (Table 1). Peri-operative CV complications, including acute myocardial infarction (MI), heart failure (HF) and CV death, rise with patients’ age (14,15), highlighting the impact of frailty and making the optimization of CV status of elderly patients with multiple comorbidities imperative.
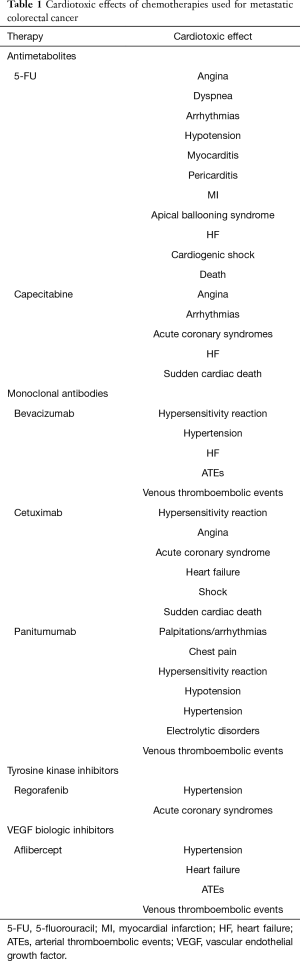
Full table
Antimetabolites
5-fluorouracil (5-FU)
Fluopyrimidines, including intravenous 5-FU and oral capecitabine, constitute the typical backbone of chemotherapeutic regimens for mCRC (11). 5-FU belongs to antimetabolites, and it is a pyrimidine analogue acting through the irreversible inhibition of thymidylate synthase. The combination of 5-FU with other cytotoxic agents, such as irinotecan and oxaliplatin, has improved the survival of patients with metastatic disease (16). The reported incidence of 5-FU cardiotoxicity varies from 1.2% to 18% (17). This significant variability can be explained by the dose and method of administration, the combination with other cardiotoxic medications, the concurrent radiotherapy and most importantly by the pre-existing CV conditions and risk factor profile.
Angina, is the most common clinical presentation of 5-FU-induced cardiotoxicity (17), followed by dyspnea, palpitations and hypotension (18). Electrocardiographic (ECG) changes such as ST segment changes, right bundle branch block (RBBB), arrhythmias, including atrial fibrillation (AF) (19), myocarditis (20) and pericarditis (21), acute MI (22), apical ballooning syndrome (23), cardiogenic shock, HF (24) and even death (25) have also been described.
Cardiotoxicity from 5-FU occurs most commonly during the first cycle (25). It can occur anytime during infusion or up to 1–2 days after, with the vast majority of cases presenting within the first 72 h and usually not later than the first 3 cycles (17). The median time to initiation of symptoms is 12 h following initiation of the infusion initiation (23). Symptoms and ECG changes may resolve soon after drug discontinuation, but cardiac complications due to 5-FU have been described up to one month after the drug discontinuation (26).
The pathogenesis of 5-FU induced cardiotoxicity has not yet been fully elucidated. Endothelial injury leading to vasoconstriction, procoagulant state and direct myocardial toxicity are the main proposed mechanisms (27). The most popular mechanism however is coronary vasospasm. Anginal pain, ST changes and troponin elevation have been developed in patients without occlusive macrovascular coronary disease (CAD) (26). A plausible explanation could be microvascular dysfunction. Dihydropyrimidine dehydrogenase (DPD) deficiency is another presumable though controversial mechanism of severe forms of 5-FU cardiotoxicity (28).
Data concerning the predisposing factors for the 5-FU induced cardiotoxicity are inconclusive regarding CV risk factors and cardiac comorbidities (25). However previous thoracic irradiation, previous treatment with cardiotoxic drugs and combination therapy with cisplatin increase the risk of developing cardiotoxicity.
The management of 5-FU cardiotoxicity depends on the clinical presentation and the severity of symptoms and comprises of 4 steps: (I) immediate discontinuation of 5-FU; (II) empirical treatment of symptoms; (III) confirmation of the causal relationship of symptoms with 5-FU and (IV) rechallenge with pharmacological prophylaxis or determination of alternative chemotherapeutic regimes. Since 5-FU cardiotoxicity can be potentially fatal, immediate discontinuation of the treatment and initiation of antianginal therapy with calcium channel blockers (CCBs) or nitrates is the only treatment suggested officially by the European Society of Cardiology (ESC) (29). This leads to improvement of symptoms in 69% of affected patients (25). In cases of severe or life-threatening CV or cerebral toxicity, uridine triacetate can be used as an antidote for fluoropyrimidines (30). This oral pyrimidine analogue of uridine, approved by the American Food and Drug Administration (FDA) in 2015, acts competing with the toxic fluorouridine triphosphate (FUTP), one of the main metabolites of 5-FU, for incorporation into RNA in normal tissues, providing protection from its toxic effects. Afterwards, it is critical to attribute the cardiac symptoms to 5-FU within reasonable degree of certainty, using mostly clinical judgment and/or laboratory tests. Unnecessary withholding of effective chemotherapy could jeopardize the patient’s chance of cure, while re-challenge could be life threatening. Obstructive CAD should be excluded with invasive or non-invasive tests according to the level of CV risk and should be treated accordingly. Finally, the use of alternative chemotherapeutic regimens is possible in mCRC, with irinotecan alone, irinotecan plus oxaliplatin, cetuximab or panitumumab (for patients with RAS/BRAF wild-type tumors) or with trifluridine-tipiracil, regorafenib and ramucirumab or raltitrexed alone or in combination regimens (31).
Capecitabine
Capecitabine, a fluoropyrimidine derivative, was developed with the aim of providing a more effective and less toxic alternate to 5-FU (32). It can substitute intravenous 5-FU as a single agent or it can be combined with oxaliplatin, with the convenience of oral administration in the first-line treatment of mCRC (11). Capecitabine is the oral prodrug of 5-FU. It can lead to prolonged exposure of tumor tissue to the active drug, mimicking continuous infusion of 5-FU, while maintaining very low plasma levels, leading possibly to significantly less serious and less frequent toxicity than 5-FU when used alone or in combination with other cytotoxic agents (32). Angina-like chest pain, acute ischemic events (33), HF and arrhythmias, such as AF (34) and/or ventricular fibrillation (35), sudden cardiac death attributed possibly to coronary vasospasm have been reported with capecitabine (36), similarly to 5-FU. The highest incidence of these events is recorded when the patients receives combined therapy with capecitabine, oxaliplatine and bevacizumab (36) and occur mostly during the first cycle (37). The management is symptomatic, while re-challenge does not seem an attractive and safe option (25). S-1, an oral fluoropyrimidine, consisting of tegafur, a 5-FU prodrug, oteracil potassium and gimeracil, which inhibits the degradation of 5-FU by DPD inhibition, can be possibly administered safely after capecitabine or 5-FU induced cardiotoxicity according to a limited number of case reports (38).
Monoclonal antibodies
Bevacizumab
Monoclonal antibodies are another class of medications that is recommended as first and second-line treatment of mCRC (11). Bevacizumab, cetuximab and panitumumab are approved for mCRC (11). Bevacizumab is the first angiogenesis inhibitor that has been approved by the FDA since 2004 for the treatment of mCRC (39). It is a humanized IgG monoclonal antibody targeting circulating vascular endothelial growth factor A ligand (VEGF-A) that plays a crucial role in regulating angiogenesis in cancer cells. It has been approved as part of combination regimens containing 5-FU/capecitabine, oxaliplatin, leucovorin and irinotecan (FOLFOX/CAPOX/FOLFIRI or FOLFOXIRI) or with fluoropyrimidine monotherapy in patients unable to tolerate aggressive treatment (11), resulting in higher median overall survival.
Cardiotoxicity of bevacizumab comprises of class effects and specific to this substance effects. A hypersensitivity reaction that can even be fatal with hypotension, dyspnea, fever and hypoxia is the most serious class effect and is due to the massive release of cytokines. The other two class effects are hypertension, the most frequent side effect of bevacizumab, and HF. The proposed responsible molecular mechanisms for the development of hypertension or the worsening of pre-existing hypertension include rarefaction of neovascularization, an imbalance in neurohormonal factors, endothelial dysfunction with reduction of vascular nitric oxide production (40) and renal dysfunction (41). Hypertension is reversible and dependent on the duration of exposure and dose (42). The timing of hypertension occurrence varies from 1 (43) to 6 months (44) after the treatment initiation. Severe hypertension (>200/110 mmHg) is reported in 5–36% of patients but cases of hypertensive emergencies with encephalopathy or subarachnoid hemorrhage are rare. Risk factors for developing bevacizumab-induced hypertension are age >65 years, body mass index (BMI) ≥25, pre-existing hypertension (45), smoking and hypercholesterolemia (46). It is suggested that routine monitoring of blood pressure (BP) must be performed every week during the first cycle, every 2–3 weeks afterwards and then at least once before every administration (41). The treatment should be individualized and usually includes angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) and dihydropyridine CCBs; diltiazem, verapamil are not recommended due to CYP3A4 inhibition, which is also the case for bevacizumab (41,45). Interestingly, it has been suggested that bevacizumab-induced hypertension may be predictive of antitumor efficacy and better survival (47), especially if occurring during the first 3 months.
HF is the third-class effect due to direct myocardial damage. Its incidence is very low in mCRC (1.3%) (48). The underlying mechanism has not been clarified, but hypertension induced by bevacizumab and the effect of blockade of VEGF to the repair of myocardial damage and collateral vessel formation may play a role (49). Standard HF treatment is applied, including neurohormonal inhibitors.
The most specific CV complication of bevacizumab is increased thromboembolic risk. Arterial thromboembolic events (ATEs) are defined by the National Cancer Institute’s Common Toxicity Criteria as myocardial ischemia or MI, cerebral infarction, cerebrovascular accident, cerebral ischemia, ischemic stroke, and peripheral or visceral ATEs (50). It is not clear whether ATEs are dose-related, as studies show controversial results (51). ATEs can occur at any time during treatment, with a median time of 3 months after treatment onset (52). Risk factors for ATEs are age >65 and past history of ATE, while prophylactic aspirin (≤375 mg) decreases the risk, without increasing bleeding events (52).
Venous thrombotic events (VTEs) induced by bevacizumab include deep vein thrombosis (DVT), pulmonary embolism (PE) and thrombophlebitis. However, the added risk of bevacizumab to VTEs is ambiguous, since cancer patients are a high-risk group (51,53). Oral anticoagulation is indicated and does not seem to increase the bleeding risk (54).
Cetuximab and panitumumab
Cetuximab and panitumumab are monoclonal antibodies that bind to the human epidermal growth factor receptor (EGFR) and can be used as first, second or third-line treatment in mCRC in different combinations in RAS-wild type and BRAF type patients (11). They can be used with 5-FU and oxaliplatin or irinotecan (FOLFOX, FOLFIRI) as first-line treatment, with 5-FU and irinotecan (FOLFIRI) as second-line and with irinotecan alone as third-line regimens (11). However, they should not be used with capecitabine or bolus 5-FU-based regimens (11). Cetuximab is a human/mouse chimeric monoclonal antibody that competitively binds to EGFR resulting in receptor internalization; it improves survival when added to other chemotherapeutic regimes (55). It can cause allergic reactions that can be severe (2–5%) and fatal (<0.1%), usually during the first hour of the first administration and sometimes also hours afterwards or during subsequent infusions. Urticaria and bronchospasm can be accompanied by hypotension and in rare cases by angina, MI, HF, shock and sudden death (56). Cetuximab may also lead to increased risk of VTEs (57) but the data are limited. Panitumumab is a fully human monoclonal IgG2 antibody produced in a mammalian cell line by recombinant DNA technology. Panitumumab has been reported to cause hypomagnesemia, hypokalemia, dehydration, hypotension, hypertension and VTEs (58). Cetuximab and panitumumab have a similar CV toxicity profile, with the most common cardiac events requiring treatment being palpitations/arrhythmias (25.8%), chest pain (8.1%), arrhythmias (4.8%) (59) and dyspnea (60).
Tyrosine kinase inhibitors (TKIs)
Regorafenib
Regorafenib is a multi-kinase inhibitor that targets VEGF receptors 1–3, KIT, PDGFR-alpha, PDGFRbeta, RET, FGFR-1 and FGFR-2, TIE2, DDR2, TrkA, Eph2A, RAF-1, BRAF, BRAFV600E, SAPK2, PTK5, and ABL, involved in angiogenesis and oncogenesis (61,62). Regorafenib is recommended in patients pre-treated with fluoropyrimidines, oxaliplatin, irinotecan, bevacizumab and in RAS-wild-type patients with EGFR antibodies, according to ESMO consensus guidelines for mCRC (11). It is relatively safe concerning CV AEs, with the most frequent being hypertension. The reported incidence rates of all-grade and high-grade hypertension are 44.4% and 12.5%, respectively (63). It is noteworthy that the incidence of hypertension is highest in cycle 1 and tapered to low or no incidence over cycles 2 to 8 (64). Hypertension typically resolves after cessation of the drug and its treatment is not based on specific evidence-based guidelines. Diuretics should be avoided as they may worsen dehydration, especially if the patient also experiences regorafenib-induced diarrhea. For resistant grade 3 hypertension, regorafenib dose should be reduced, while for grade 4, regorafenib should be held till BP levels return to grade 2 or normal (65). However preventive strategy is more important, and it is crucial to identify and treat hypertension before initiation of regorafenib therapy. Then BP monitoring by a healthcare professional at a weekly basis is useful for the first two cycles of therapy. Thereafter, patients should be encouraged to measure their BP at home daily (65).
Myocardial ischemia and infarction are rare adverse cardiac effects of regorafenib by unclear pathophysiological mechanisms (64).
Newer agents
Trifluridine/tipiracil (TAS-102) belongs to fluoropyrimidines and is another new option in patients with mCRC and pre-existing cardiac disease, pretreated with all active drugs and biologics, that can be used instead of regorafenib with better safety profile (11,66). TAS-102, is well tolerated and does not appear to have any cardiac toxicity (31,66).
Aflibercept also known as ziv-aflibercept or VEGF-Trap, is an intravenously administered, fully human, recombinant fusion protein that blocks the activity of soluble VEGF-A, VEGF-B, and PIGF (placental growth factor) by acting as a ligand trap, thus inhibiting the growth of new blood vessels that supply oxygen and nutrients to tumors dependent on VEGF pathways (67). Aflibercept is approved in combination with FOLFIRI as second line treatment in mCRC that is resistant to or has progressed after treatment with an oxaliplatin-containing regimen (68). It is associated with significantly longer progression-free survival with acceptable tolerability (69). The most frequent cardiotoxic effect of aflibercept as a VEGF inhibitor is hypertension (69), while HF, ATEs and VTEs are uncommon (70). The incidence of all-grade hypertension ranges between 16.7% and 51.4%, while of high-grade hypertension from 6.3% to 27.3% (71). Half of the cases of aflibercept-induced hypertension develop during cycles 1–5 (70) and there is no association with the BP levels before its administration (72). Since uncontrolled, severe hypertension induced by aflibercept could lead to serious CV complications such as hypertensive encephalopathy, central nervous system hemorrhage, HF and ATEs (73), early diagnosis and treatment is essential for outcome. Blood pressure monitoring after initiation of therapy with aflibercept is almost obligatory, but there are no specific guidelines concerning treatment. Permanent discontinuation of aflibercept is suggested in patients with hypertensive crisis or hypertensive encephalopathy and temporal suspension in cases with severe hypertension that is not controlled with medical management (71).
Newer therapeutic options, such as the combination of veliparib, a poly (adenosine diphosphate ribose) polymerase (PARP) inhibitor and temozolomide, a potent DNA-alkylating agent is well tolerated in patients with advanced, refractory to all the aforementioned therapies mCRC (74) with good tolerability and safety profile.
Cardio-oncology consultation
Figure 1 outlines the scheme of cardio-oncology consultation in patients with mCRC. The main aims of cardio-oncology consultation are to render the cancer patient fit to receive the indicated cancer therapy, to minimize the risk of cardiotoxicity, and if cardiotoxicity occurs, to diagnose it early to allow its proper and timely management. Patients with risk factors for cardiotoxicity are referred to the Cardio-oncology clinic before cancer therapy (75). Baseline assessment at the Cardio-oncology clinic consists of clinical evaluation and ECG and, if deemed necessary, echocardiography and cardiac biomarkers (troponin and/or natriuretic peptides) to identify possible CV disease or risk factors and to stratify the risk of cardiotoxicity. If necessary, treatment of CV disease or modification of risk factors is applied. The type and frequency of subsequent Cardio-oncology monitoring during and after cancer is scheduled according to initial risk stratification.

Conclusions
The overall survival of patients with mCRC has been significantly improved over the last years from approximately one year to more than 30 months in recent clinical trials with the integration of multiple cytotoxic agents and targeted therapies. Some of these regimens may lead to the development of cardiotoxic events, jeopardizing patients’ life, quality of life and chance to survive if therapy has to be discontinued. The earlier recognition of CV AEs is a key element to proper treatment and to suitable adjustment of the regimen used, if needed.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Towler BP, Irwig L, Glasziou P, et al. Screening for colorectal cancer using the faecal occult blood test, hemoccult. Cochrane Database Syst Rev 2000.Cd001216. [PubMed]
- Stewart BW, Wild CP. World Cancer Report 2014. IARC Nonserial Publication. WHO Press, World Health Organization, 2014.
- Haraldsdottir S, Einarsdottir HM, Smaradottir A, et al. Colorectal cancer - review. Laeknabladid 2014;100:75-82. [PubMed]
- Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence – SEER 18 Regs Research Data with Delay-Adjustment, Malignant Only, Nov 2015 Sub (2000-2013). Available online: https://seer.cancer.gov/data-software/documentation/seerstat/nov2015/
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Elferink MA, de Jong KP, Klaase JM, et al. Metachronous metastases from colorectal cancer: a population-based study in North-East Netherlands. Int J Colorectal Dis 2015;30:205-12. [Crossref] [PubMed]
- Riihimaki M, Hemminki A, Sundquist J, et al. Patterns of metastasis in colon and rectal cancer. Sci Rep 2016;6:29765. [Crossref] [PubMed]
- Merkel S, Weber K, Croner RS, et al. Distant metastases in colorectal carcinoma: A proposal for a new M1 subclassification. Eur J Surg Oncol 2016;42:1337-42. [Crossref] [PubMed]
- Fakih MG. Metastatic colorectal cancer: current state and future directions. J Clin Oncol 2015;33:1809-24. [Crossref] [PubMed]
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386-422. [Crossref] [PubMed]
- Benson AB 3rd, Venook AP, Al-Hawary MM, et al. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J Natl Compr Canc Netw 2018;16:359-69. [Crossref] [PubMed]
- Latremouille-Viau D, Chang J, Guerin A, et al. The economic burden of common adverse events associated with metastatic colorectal cancer treatment in the United States. J Med Econ 2017;20:54-62. [Crossref] [PubMed]
- Jafari MD, Jafari F, Halabi WJ, et al. Colorectal Cancer Resections in the Aging US Population: A Trend Toward Decreasing Rates and Improved Outcomes. JAMA Surg 2014;149:557-64. [Crossref] [PubMed]
- Aquina CT, Mohile SG, Tejani MA, et al. The impact of age on complications, survival, and cause of death following colon cancer surgery. Br J Cancer 2017;116:389-97. [Crossref] [PubMed]
- Sanchez-Gundin J, Fernandez-Carballido AM, Martinez-Valdivieso L, et al. New Trends in the Therapeutic Approach to Metastatic Colorectal Cancer. Int J Med Sci 2018;15:659-65. [Crossref] [PubMed]
- Becker K, Erckenbrecht JF, Haussinger D, et al. Cardiotoxicity of the antiproliferative compound fluorouracil. Drugs 1999;57:475-84. [Crossref] [PubMed]
- Polk A, Vaage-Nilsen M, Vistisen K, et al. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev 2013;39:974-84. [Crossref] [PubMed]
- Stewart T, Pavlakis N, Ward M. Cardiotoxicity with 5-fluorouracil and capecitabine: more than just vasospastic angina. Intern Med J 2010;40:303-7. [Crossref] [PubMed]
- Dalzell JR, Samuel LM. The spectrum of 5-fluorouracil cardiotoxicity. Anticancer Drugs 2009;20:79-80. [Crossref] [PubMed]
- Calik AN, Celiker E, Velibey Y, et al. Initial dose effect of 5-fluorouracil: rapidly improving severe, acute toxic myopericarditis. Am J Emerg Med 2012;30:257.e1-3. [Crossref] [PubMed]
- Lestuzzi C, Vaccher E, Talamini R, et al. Effort myocardial ischemia during chemotherapy with 5-fluorouracil: an underestimated risk. Ann Oncol 2014;25:1059-64. [Crossref] [PubMed]
- Basselin C, Fontanges T, Descotes J, et al. 5-Fluorouracil-induced Tako-Tsubo-like syndrome. Pharmacotherapy 2011;31:226. [Crossref] [PubMed]
- Robben NC, Pippas AW, Moore JO. The syndrome of 5-fluorouracil cardiotoxicity. An elusive cardiopathy. Cancer 1993;71:493-509. [Crossref] [PubMed]
- Saif MW, Shah MM, Shah AR. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin Drug Saf 2009;8:191-202. [Crossref] [PubMed]
- Tsibiribi P, Descotes J, Lombard-Bohas C, et al. Cardiotoxicity of 5-fluorouracil in 1350 patients with no prior history of heart disease. Bull Cancer 2006;93:E27-30. [PubMed]
- Depetris I, Marino D, Bonzano A, et al. Fluoropyrimidine-induced cardiotoxicity. Crit Rev Oncol Hematol 2018;124:1-10. [Crossref] [PubMed]
- Shahrokni A, Rajebi MR, Harold L, et al. Cardiotoxicity of 5-fluorouracil and capecitabine in a pancreatic cancer patient with a novel mutation in the dihydropyrimidine dehydrogenase gene. JOP 2009;10:215-20. [PubMed]
- Zamorano JL, Lancellotti P, Rodriguez Munoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768-801. [Crossref] [PubMed]
- Ison G, Beaver JA, McGuinn WD Jr, et al. FDA Approval: Uridine Triacetate for the Treatment of Patients Following Fluorouracil or Capecitabine Overdose or Exhibiting Early-Onset Severe Toxicities Following Administration of These Drugs. Clin Cancer Res 2016;22:4545-9. [Crossref] [PubMed]
- Vaflard P, Ederhy S, Torregrosa C, et al. Bull Cancer 2018;105:707-19. [Fluoropyrimidines cardiac toxicity: 5-fluorouracil, capecitabine, compound S-1 and trifluridine/tipiracil]. [Crossref] [PubMed]
- McKendrick J, Coutsouvelis J. Capecitabine: effective oral fluoropyrimidine chemotherapy. Expert Opin Pharmacother 2005;6:1231-9. [Crossref] [PubMed]
- Senturk T, Kanat O, Evrensel T, et al. Capecitabine-induced cardiotoxicity mimicking myocardial infarction. Neth Heart J 2009;17:277-80. [Crossref] [PubMed]
- Leicher LW, de Graaf JC, Coers W, et al. Tolerability of Capecitabine Monotherapy in Metastatic Colorectal Cancer: A Real-World Study. Drugs R D 2017;17:117-24. [Crossref] [PubMed]
- Shah NR, Shah A, Rather A. Ventricular fibrillation as a likely consequence of capecitabine-induced coronary vasospasm. J Oncol Pharm Pract 2012;18:132-5. [Crossref] [PubMed]
- Kwakman JJ, Simkens LH, Mol L, et al. Incidence of capecitabine-related cardiotoxicity in different treatment schedules of metastatic colorectal cancer: A retrospective analysis of the CAIRO studies of the Dutch Colorectal Cancer Group. Eur J Cancer 2017;76:93-9. [Crossref] [PubMed]
- Ng M, Cunningham D, Norman AR. The frequency and pattern of cardiotoxicity observed with capecitabine used in conjunction with oxaliplatin in patients treated for advanced colorectal cancer (CRC). Eur J Cancer 2005;41:1542-6. [Crossref] [PubMed]
- Franck C, Malfertheiner P, Venerito M. Safe administration of S-1 after 5-fluorouracil-induced cardiotoxicity in a patient with colorectal cancer. BMJ Case Rep 2017;2017.
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [Crossref] [PubMed]
- Di Lorenzo G, Autorino R, Bruni G, et al. Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: a multicenter analysis. Ann Oncol 2009;20:1535-42. [Crossref] [PubMed]
- Brinda BJ, Viganego F, Vo T, et al. Anti-VEGF-Induced Hypertension: a Review of Pathophysiology and Treatment Options. Curr Treat Options Cardiovasc Med 2016;18:33. [Crossref] [PubMed]
- Zhu X, Wu S, Dahut WL, et al. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: systematic review and meta-analysis. Am J Kidney Dis 2007;49:186-93. [Crossref] [PubMed]
- Osterlund P, Soveri LM, Isoniemi H, et al. Hypertension and overall survival in metastatic colorectal cancer patients treated with bevacizumab-containing chemotherapy. Br J Cancer 2011;104:599-604. [Crossref] [PubMed]
- Nakaya A, Kurata T, Yokoi T, et al. Retrospective analysis of bevacizumab-induced hypertension and clinical outcome in patients with colorectal cancer and lung cancer. Cancer Med 2016;5:1381-7. [Crossref] [PubMed]
- Wasserstrum Y, Kornowski R, Raanani P, et al. Hypertension in cancer patients treated with anti-angiogenic based regimens. Cardio-Oncology 2015.1.
- Small HY, Montezano AC, Rios FJ, et al. Hypertension due to antiangiogenic cancer therapy with vascular endothelial growth factor inhibitors: understanding and managing a new syndrome. Can J Cardiol 2014;30:534-43. [Crossref] [PubMed]
- Cai J, Ma H, Huang F, et al. Correlation of bevacizumab-induced hypertension and outcomes of metastatic colorectal cancer patients treated with bevacizumab: a systematic review and meta-analysis. World J Surg Oncol 2013;11:306. [Crossref] [PubMed]
- Tahover E, Hubert A, Temper M, et al. An observational cohort study of bevacizumab and chemotherapy in metastatic colorectal cancer patients: safety and efficacy with analysis by age group. Target Oncol 2015;10:55-63. [Crossref] [PubMed]
- Verma N, Swain SM. Bevacizumab and heart failure risk in patients with breast cancer: a thorn in the side? J Clin Oncol 2011;29:603-6. [Crossref] [PubMed]
- National Cancer Institute’s Common Toxicity Criteria (version 4). Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf
- Totzeck M, Mincu RI, Rassaf T. Cardiovascular Adverse Events in Patients With Cancer Treated With Bevacizumab: A Meta-Analysis of More Than 20 000 Patients. J Am Heart Assoc 2017.6. [PubMed]
- Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst 2007;99:1232-9. [Crossref] [PubMed]
- European Medicines Agency. Avastin Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/erbitux-epar-product-information_en.pdf
- Hurwitz HI, Saltz LB, Van Cutsem E, et al. Venous thromboembolic events with chemotherapy plus bevacizumab: a pooled analysis of patients in randomized phase II and III studies. J Clin Oncol 2011;29:1757-64. [Crossref] [PubMed]
- Van Cutsem E, Kohne CH, Lang I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011;29:2011-9. [Crossref] [PubMed]
- Baron Esquivias G, Asteggiano R. Cardiac Management of Oncology Patients: Clinical Handbook for Cardio-Oncology. New York: Springer International Publishing; 2015.
- Petrelli F, Cabiddu M, Borgonovo K, et al. Risk of venous and arterial thromboembolic events associated with anti-EGFR agents: a meta-analysis of randomized clinical trials. Ann Oncol 2012;23:1672-9. [Crossref] [PubMed]
- Hecht JR, Cohn A, Dakhil S, et al. SPIRITT: A Randomized, Multicenter, Phase II Study of Panitumumab with FOLFIRI and Bevacizumab with FOLFIRI as Second-Line Treatment in Patients with Unresectable Wild Type KRAS Metastatic Colorectal Cancer. Clin Colorectal Cancer 2015;14:72-80. [Crossref] [PubMed]
- Tang XM, Chen H, Liu Y, et al. The cardiotoxicity of cetuximab as single therapy in Chinese chemotherapy-refractory metastatic colorectal cancer patients. Medicine (Baltimore) 2017;96:e5946. [Crossref] [PubMed]
- Tang XM, Chen H, Li Q, et al. Assessment of the cardiac safety between cetuximab and panitumumab as single therapy in Chinese chemotherapy-refractory mCRC. Onco Targets Ther 2017;11:123-9. [Crossref] [PubMed]
- Mross K, Frost A, Steinbild S, et al. A phase I dose-escalation study of regorafenib (BAY 73-4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res 2012;18:2658-67. [Crossref] [PubMed]
- Kimmick GG, Lenihan DJ, Sawyer DB, et al. Cardio-Oncology: The Clinical Overlap of Cancer and Heart Disease. New York: Springer International Publishing; 2017.
- Wang Z, Xu J, Nie W, et al. Risk of hypertension with regorafenib in cancer patients: a systematic review and meta-analysis. Eur J Clin Pharmacol 2014;70:225-31. [Crossref] [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [Crossref] [PubMed]
- Krishnamoorthy SK, Relias V, Sebastian S, et al. Management of regorafenib-related toxicities: a review. Therap Adv Gastroenterol 2015;8:285-97. [Crossref] [PubMed]
- Petrelli F, Barni S, Bertocchi P, et al. TAS-102, the first "cardio-gentle" fluoropyrimidine in the colorectal cancer landscape? BMC Cancer 2016;16:386. [Crossref] [PubMed]
- Syed YY, McKeage K. Aflibercept: A Review in Metastatic Colorectal Cancer. Drugs 2015;75:1435-45. [Crossref] [PubMed]
- National Institute for Health and Care Excellence. Aflibercept in combination with irinotecan and fluorouracil-based therapy for treating metastatic colorectal cancer that has progressed following prior oxaliplatin-based chemotherapy. Available online: http://www.nice.org.uk
- Pentheroudakis G, Kotoula V, Koliou GA, et al. AMALTHEA: Prospective, Single-Arm Study of the Hellenic Cooperative Oncology Group (HeCOG) Evaluating Efficacy and Safety of First-Line FOLFIRI + Aflibercept for 6 Months Followed by Aflibercept Maintenance in Patients With Metastatic Colorectal Cancer. Clin Colorectal Cancer 2018;17:e631-7. [Crossref] [PubMed]
- Pastorino A, Di Bartolomeo M, Maiello E, et al. Aflibercept Plus FOLFIRI in the Real-life Setting: Safety and Quality of Life Data From the Italian Patient Cohort of the Aflibercept Safety and Quality-of-Life Program Study. Clin Colorectal Cancer 2018;17:e457-70. [Crossref] [PubMed]
- Qi WX, Shen Z, Tang LN, et al. Risk of hypertension in cancer patients treated with aflibercept: a systematic review and meta-analysis. Clin Drug Investig 2014;34:231-40. [Crossref] [PubMed]
- Giuliani J, Bonetti A. The development of hypertension in metastatic colorectal cancer patients treated with aflibercept: the role of systolic and diastolic blood pressure before starting treatment. Recenti Prog Med 2016;107:199-200. [PubMed]
- Rougier P, Riess H, Manges R, et al. Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. Eur J Cancer 2013;49:2633-42. [Crossref] [PubMed]
- Pishvaian MJ, Slack RS, Jiang W, et al. A phase 2 study of the PARP inhibitor veliparib plus temozolomide in patients with heavily pretreated metastatic colorectal cancer. Cancer 2018;124:2337-46. [Crossref] [PubMed]
- Farmakis D, Keramida K, Filippatos G. How to build a cardio-oncology service? Eur J Heart Fail 2018;20:1732-4. [Crossref] [PubMed]


