Endoscopic stenting in colorectal cancer
Introduction
Colorectal cancer (CRC) is one of the most common cancer in the world (1). Large bowel obstruction is one of the few emergency presentations of CRC. Studies have showed that the proportion of CRC patients admitted to hospital as an acute emergency case hover around 10–30% for the past 5 years (2-4).
Emergency surgery for acute malignant large bowel obstruction is associated with morbidity rates of 40–60% and mortality of 3–11% (5-7). Peri-operative complications such as death, sepsis, anastomotic leaks, wound infection and cerebrovascular accidents are usually encountered, with the addition of higher rates of permanent stoma formation (8).
The use of self-expanding metallic stents (SEMS) was first described for the management of acute large bowel obstruction since the early 1990s by Tejero et al. (9). The use of SEMS has progressed from palliating advanced CRC and as a means to bridge the need for emergent surgery to an elective one (10).
Since then, the short- and long-term outcomes of endoscopic colonic stenting have been reviewed with its safety profile being determined to minimize any potential harm to any patient receiving this form of treatment. For patients with acute large bowel obstruction presenting with metastatic disease, we have to evaluate the role endoscopic colonic stenting play in palliating the obstruction. Numerous randomized controlled trials were conducted comparing the use of SEMS as a bridge to surgery versus emergency surgery for the management of acute malignant large bowel obstruction (11-17). The rationale for such a move is to provide swift and effective decompression of the large bowel, minimize the risk of bacterial translocation, whilst allowing for proper oncological staging, physiological optimization and resuscitation before having the patient undergo surgery in an elective setting.
Numerous studies have been performed to evaluate the role of SEMS in malignant large bowel obstruction, however several of them highlighted contrasting results. Hence, we conducted this review article to evaluate the evidence of the role of SEMS in the management of patients with acute malignant large bowel obstruction.
Patient selection
The management of patients presenting with acute malignant left-sided large bowel obstruction, including the rectum should be individualized. Proper patient selection is crucial in ensuring good outcome. The group of patients who may be affected by this condition are usually older and hence a comprehensive review of their medical comorbidities is of particular importance to determine their fitness for surgery (7). Studies have reported that almost 70–80% of large bowel obstruction are actually located in the left side of the colon, which makes them amenable to endoscopic interventions (18,19). Of which about 10% of these patients will require emergency surgery and are associated with poor outcomes with morbidity as high as 70% and mortality reaching 12% (7,20). Compared to the morbidity levels of elective surgery for CRC which are reported to be less than 5% (21).
Options for treatment may include staged surgical resection with or without anastomosis (e.g., Hartmann resection), resection of the distended bowel (e.g., subtotal/total colectomy), or temporary relief of obstruction and faecal load (e.g., creation of proximal defunctioning loop colostomy or ileostomy or the use of endoscopic stenting through the stenosed segment as a bridge to surgery in an elective setting). A tumour in the lower rectum cannot be stented as the distal end of the stent will causes tenesmus and faecal incontinence giving rise to poorer quality of life (22). We need to consider the patient factor, disease factor through CT scan (site of obstruction, length of stenosis, presence of metastasis, perforation) and available expertise, which will be further elaborated in this review. Each of these options has advantages and disadvantages and the ultimate decision must be made with the best interest of the patient and the clinical presentation in mind (23).
Overview of self-expanding metallic stents
There are no large-scale studies to determine the superiority of the SEMS that are currently in the market. Variables such as material, design, diameter, length, flexibility, foreshortening ratio and delivery system are considered when selecting appropriate SEMS for each individual patient (23-27).
There are many endoscopic colonic stents available in the market. Majority of the stents are made of nitinol (alloy of nickel and titanium) which enables good flexibility and elasticity allowing for smooth deployment (28,29). Examples of which are Ultraflex (Boston Scientific, Natick, MA, USA) and Alimaxx E (Alveolus, Charlotte, NC, USA) stents. Other materials include stainless steel (i.e., Z-stent) (Cook Medical, Bloomington, IN, USA) and Elgiloy (alloy of cobalt, chromium and nickel) (i.e., Wallstent) (Boston Scientific, Natick, MA, USA) (29). Colonic stents can be broadly divided into two main groups: uncovered and covered. Recent meta-analysis comparing the technical success rates of these two groups showed no significant difference (29). The studies also reported similar stent migration rates (30,31). However, the benefit conferred by inserting a covered colonic stent is the association with higher tumour in-growth (RR 6; 95% CI: 2.23–16.1, P=0.0004) (30,32). This is an important aspect to consider when performing palliative endoscopic colonic stenting, in order to avoid having to go through another intervention subsequently.
Description of the endoscopic colonic stenting procedure using SEMS
The procedure is carried out endoscopically under image intensifier guidance at the endoscopy suite, the fluoroscopy room at the department of diagnostics imaging using portable endoscopic equipment, or the operating theatre (especially if there is a hybrid theatre for endovascular procedures). Access to an operating facility is required as abdominal distention secondary to gas insufflation during the stenting procedure can lead to inadvertent perforation, necessitating immediate transfer to the operating theatre (33).
When possible, ensure that the patient receives a rectal enema for clearance of the bowel distal to the obstruction before stenting to facilitate scope insertion (34). Prior to the procedure, the patient is positioned in the left lateral position under conscious sedation. A double lumen colonoscope is preferred as it facilitates simultaneous suction and irrigation via one channel whilst advancing the guidewire in the other channel. If available, carbon dioxide insufflation should be used in preference to room air.
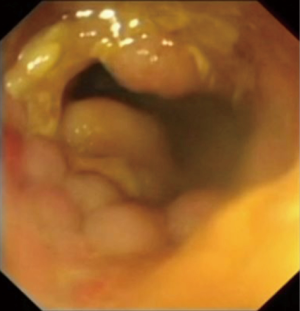
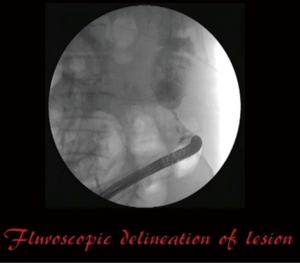
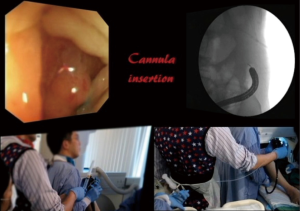
The distal end of the stenosing malignant lesion will be encountered (Figure 1). Identify any area on the tumour that will allow passage of the lipophilic guidewire into the proximal bowel, and advance the guidewire under fluoroscopy (Figure 2). Multiple attempts of passing the guidewire may be necessary. The cannula is then inserted over the guidewire using the Seldinger technique into the proximal bowel to allow injection of water soluble radiological contrast to establish the proximal and distal extent of the tumour using fluoroscopy (Figure 3). The length of the tumour is measured before deciding on the optimal length of stent to be used. There needs to be sufficient stent overhang on both ends of the tumour to minimize migration of the stent after full expansion.
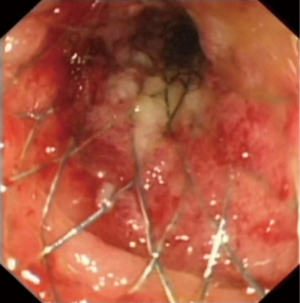
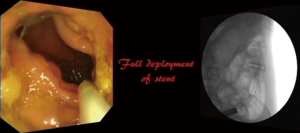
The cannula is then exchanged for the stent in its deploying device, whilst keeping the guidewire in its original position. Frequent confirmation with fluoroscopy during this step is required. The stent deploying device is advanced under direct fluoroscopy and endoscopic visualization until the entire stent is deployed. There is a need for counter-traction on the device during the deployment phase as there is a tendency for the stent to be drawn into the proximal bowel due to the radial expansion of the stent (Figure 4) (35). Simultaneous monitoring with fluoroscopy and direct vision via endoscopy is mandatory (Figure 5). Once the stent is fully deployed successfully (Figure 6), its position is confirmed under fluoroscopy. The distal end of the stent should be visible beyond the malignant lesion, and a gush of faeculent material should be encountered (Figure 7).
The patient is usually monitored overnight and an abdominal X-ray is taken within the next 24 hours to confirm the position and expansion of the stent. A vigilant look out for immediate post-procedural complications such as perforation is required. Oral intake can resume once the abdominal distension resolves. The patient is routinely prescribed stool softeners and a low residue diet, to enable ease of passage of stools. Most colonic stents expand to a diameter of 25 mm as described above, hence high dietary fibre can theoretically cause obstruction of the stent (32).
Short-term outcomes of the use of SEMS in endoscopic colonic stenting
There are numerous randomized controlled trials and systematic reviews that have compared endoscopic colonic stenting versus emergency surgery for acute malignant left-sided large bowel obstruction (4,5,8,10,35). Endoscopic colonic stenting has been recognized as a safe and effective means to alleviate acute large bowel obstruction and act as a bridge to curative surgery performed in an elective setting. The role of pre-operative stenting in the emergent management of acute malignant large bowel obstruction has been supportive by several pooled analyses that demonstrate efficacy and safety and cost-effectiveness analysis studies as described (36,37).
Predictors for failed SEMS deployment
There are some technical considerations that the endoscopist should note before undertaking the procedure to achieve a successful stent deployment. Technical failure can be defined in the following scenarios: (I) inability to cannulate the guide wire through the tumour, and (II) unsuccessful stent deployment. Clinical failure occurs when there is a failure of the deployed stent to relieve the obstruction, often due to inadequate expansion requiring subsequent surgical decompression. It is reported in systemic reviews that the technical success rates range from 80–90%, while the clinical success rates hovers around 70–80% (38). Little et al. observed that technical success rates decreased when the onset of symptoms is more than 1 week resulting in a drop from 85.4% to 69.6% (39).
Patients who failed endoscopic colonic stenting and had to undergo subsequent emergent surgery are 3 times (OR 3.3; 95% CI: 1.19–9.20; P=0.026) more likely to experience worse outcomes compared to those who had emergency surgery upfront (40). To avoid such morbidities, the suitability for stenting has to be evaluated on CT scan including anatomical considerations (e.g., tumour location and length of the stricture) and availability of adequate expertise.
Firstly, an evaluation of the length of stenosis has to be performed. Failures are more frequently encountered in cases where the stenosis is longer than 4 cm due to inadequate stent expansion (34,39). The recommended stent length should be sufficient to bridge the stenosed segment and provide an overhang of at least 2 cm on each side of the malignant lesion (19,41). What is promising is the use of multiple stents to bridge a long segment of obstruction or the presence of synchronous lesions by a few case reports to overcome this issue (28).
Next, the angulation of the lumen present at the area of malignant stenosis. Acute angulations of more than 165 degrees between the malignant lesion and distal lumen is associated with higher failure rates (40). Such acute angulations are postulated to be due to invasion of the underlying structures, making the stenting procedure more challenging and occasionally impossible (39). Thirdly, the degree of stenosis of the malignant lesion. It is reported that the risk of bowel perforation increases by 7 times (OR 6.88; 95% CI: 2.0–23.2, P=0.002) in the presence of complete large bowel obstruction compared to those with sub-total obstruction (42). CT imaging may reveal the presence of caecal pneumatosis for patients with acute large bowel obstruction. A review has shown that not all caecal pneumatosis is associated with non-viable caecum and its presence does not pose as an absolute contra-indication to endoscopic stenting (43).
Next, extra-colonic origin of large bowel obstruction can disrupt the colonic luminal patency (44). Overall technical success for the use of SEMS in these instances range from 42% to 100% and clinical success rates of 25–87.5% (45-50). Despite these success rates, it is observed that the luminal patency is lower in those with extra-colonic malignancies compared to those with intrinsic malignancies (51).
Lastly, there is a steep learning curve for the proceduralist performing the stenting procedure. Its availability depends on local expertise and the availability of fluoroscopy, as well as the specialized endoscopic equipment for the stent placement. The level of expertise present in the institution has a direct correlation to the rates of successful stenting and complications (52,53). To increase the chances for success, the proceduralist is expected to have attempted more than 20 procedures and be familiar with other endoscopic procedures like the endoscopic retrograde cholangiopancreatography (ERCP) (54,55).
Outcomes of successful SEMS deployment
Outcomes such as clinical success rates, peri-operative outcomes and rates of primary anastomosis are commonly reviewed. In a comparison between endoscopic colonic stenting and emergency surgery, the stenting procedure confers superior clinical success rates (98.6% vs. 78.1%) with similar post intervention outcomes (56). Thirty-day mortality (2.3%) was reported to be low, with similar overall complication rates [33.1–39.2% (stent) vs. 45.7–53.9% (emergency surgery)] in both groups (56,57). The preferable outcomes led to shorter length of hospital stay in the stenting group (6 vs. 8 days, P=0.028) (15), making it a more cost-effective option than emergency surgery during the initial hospitalization stay (18).
During the subsequent elective surgery, laparoscopic approach can be attempted to reap benefits such as lower rates of ileus, lesser pain, shorter duration of analgesia use, and length of hospital stay (58). Large bowel obstruction used to be considered as a relative contraindication to laparoscopic approach given the poor surgical field that could be encountered due to the presence of distended bowel and likelihood of bowel injury (59). However, the use of SEMS can improve the eventual surgical field by decompressing the distended bowel and reducing the degree of bowel oedema prior to the elective surgery which facilitates primary anastomosis (60).
Studies have reported higher primary anastomosis rates (RR 1.58; 95% CI: 1.22–2.04, P<0.001) (57). However, in a systematic review and meta-analysis performed by Cirocchi et al., there was no difference in primary anastomosis rates between the stenting and surgery group (37). But the non-randomised studies seem to suggest that endoscopic colonic stenting facilitates the occurrence of one-stage surgical intervention (67.2% vs. 55.1%, P<0.01) (34,57), with success rates for single stage elective surgery to be 60–85% (53,61). There was no significant difference between the two groups regarding anastomotic leakage (4.1% vs. 5.9%) (OR 0.74; 95% CI: 0.33–1.67, P=0.47) and intra-abdominal infection (1.4% vs. 3.2%) (OR 0.62; 95% CI: 0.12–3.19, P=0.57) (62,63).
In a meta-analysis that was performed by Allievi et al., endoscopic colonic stenting as a bridge to surgery appears to be a safe approach with advantages such as reducing the incidence of peri-operative complication rates (37.8% vs. 54.9%) and lower stoma rates (28.8% vs. 46%), with no difference in overall mortality rates (10%) (57,62,64). This was concurred by Arezzo et al. who reported in meta-analysis that the use of SEMS as a bridge to elective surgery is associated with lower overall morbidity (33.9% vs. 51.2%, P=0.03) and rates of temporary (33.9% vs. 51.4%) (65) and permanent (9% vs. 27.4%, P<0.01) stoma creation (34).
Stent-related complications
Complications arising from the insertion of SEMS can be divided by the degree of severity (minor or major), and early (≤30 days) or late (>30 days). The risk of endoscopic colonic stenting will have to be discussed with the patient prior to the procedure. It is advisable to obtain consent of the patient for possible surgical intervention in the event that the stenting is not successful. Major complications such as stent perforation (4–8%), migration (3–10%), and re-obstruction from tumour in-growth (3–10%) have to be explained (9,39,57), while minor complications include bleeding, pain, tenesmus and incontinence described (10,36).
Major complication—perforation
Initial clinical trials performed by the Dutch raised concerns over the safety of endoscopic colonic stenting, which led to early termination of the trial given high perforation and anastomotic leak rates (17). Postulation for the high perforation rates was associated with balloon pre-dilation; something my institution does not practice (66). We have to be mindful that despite reports of clinical perforation rates hovering around 7% but a histological analysis of surgical specimens revealed higher perforation rates of up to 14% (62). Increased risk of perforation is observed when anti-angiogenic agents like bevacizumab is used (9). However, recent studies have recognized colonic stenting as an accepted treatment approach for obstructing left sided colonic malignancy particularly as a bridge to palliative therapy (64). Stent related perforation can occur immediately or delayed. Immediate causes of perforation include wire or catheter misplacement. While, predictors for delayed perforation include the presence of thin-walled caecum, placement of SEMS at the recto-sigmoid junction with sharp angulation and excessive amount of air insufflation in a distended large bowel (28,67,68).
Major complication—stent migration and re-obstruction
Stent migration rates of covered stents (8–50%) exceeds uncovered stents (3–36%) (69). This occurs when the stent diameter is too narrow or too short in length in comparison with the obstructing segment, or if the lesion shrinks after chemotherapy (70). Stent re-obstruction occurs over time if the cancer is not removed. Treatment options include surgery or repeated stenting (71). New strategies will have to be in place to avoid stent-related complications and prolong stent patency (35). With the development of new material and design, or the presence of drug eluding stents, we can reduce the chance of developing stent migration and re-obstruction. In one animal study that validated the usefulness of 5-fluorouracil-loaded polydioxanone stent for the treatment of CRC, in-stent re-stenosis was reduced by 50% (6.4% vs. 12.8%) (72).
Minor complications
Bleeding can usually be treated conservatively, with pain relief provided through the use of analgesia. Faecal incontinence can be avoided if the SEMS is placed at least 2 cm proximal to anal verge (68).
Long-term outcomes of the use of SEMS in endoscopic colonic stenting
The literature is still divided regarding the long-term outcomes of endoscopic colonic stenting. There were three studies from the west which reported lower overall survival rates for patients who underwent endoscopic colonic stenting with a higher 5-year cancer specific mortality (48% vs. 21%) (73). On the other hand, other studies showed no difference in overall survival (97.8% vs. 94.3%, P=0.469) and 5-year disease free survival (79.6% vs. 70.2%, P=0.218) given similar uptake of adjuvant chemotherapy and lymph node harvested (74,75).
In a prospective cohort study by Gorissen et al. it showed higher local recurrence rates (32% vs. 8%, P=0.027) in patients who received endoscopic colonic stenting (74). A postulation for observed increased in local recurrence rates (73) is the presence of higher rates of peri-neural invasion in the histopathological assessment of the stented colonic segments (4). The pressure effect exerted by the SEMS could potentially have induced tumour cell invasion into the nerves via the dissemination of cancer cells during the procedure (76). A predictor for higher loco-regional recurrence and distant metastasis rates was also associated with the presence of stent related perforation as observed in a study by Sloothaak et al. (83% stent with perforation vs. 28% surgery only vs. 40% stent without perforation). This lead to worse disease free survival rates in the subgroup with stent related perforation (0% vs. 45%, P=0.007) (77).
The prognostic impact of endoscopic colonic stenting remains unclear (57,74,78), further studies will be required to determine its impact on overall survival and disease free survival in this population (11,13,77,79). Current literature seem to suggest that SEMS is a good treatment option to palliate patients with obstructed colonic anastomosis sites due to cancer recurrences (29).
Outcomes of patients receiving treatment for palliative intent
Endoscopic colonic stenting using SEMS represents an alternative to colostomy for patients with inoperable malignant colonic lesions presenting with large bowel obstruction (80). The European Society of Gastrointestinal Endoscopy (ESGE) recommends that endoscopic colonic stenting using SEMS is the preferred treatment for palliation of malignant large bowel obstruction (34). Two systematic reviews were performed comparing palliative endoscopic colonic stenting versus emergent surgery (81,82). The benefits of endoscopic colonic stenting performed in patients with metastatic large bowel obstruction include shorter hospital stay (5 vs. 12 days, P=0.003), earlier initiation of chemotherapy (4 vs. 7 weeks, P=0.02), and lower stoma formation rates (OR 0.19; 95% CI: 0.12–0.28), P<0.01) (82,83).
Patients should also be counselled on the possible stent related complications that can occur, such as perforation (8–10%), migration (8.4–9.2%) and re-obstruction (13.1–18.3%) (83). Patients managed with palliative endoscopic colonic stenting can be treated safely with chemotherapy without anti-angiogenic agents as recommended by ESGE (34). This is because several retrospective studies have showed an increase risk of perforation when bevacizumab is used (84,85).
The importance of chemotherapy after surgery in metastatic CRC cannot be understated (86-90). Although the stenting procedure itself does not confer any survival benefit (7.6 vs. 7.8 months) (82), it increases the possibility of down-staging previously unresectable metastatic disease (91). To allow these patients the chance to have better long-term outcome, the ability to administer chemotherapy within a certain therapeutic window is important, beyond which, the benefits are questionable as the role of palliative endoscopic colonic stenting in patients who have resectable metastasis is unclear (92). What is crucial for these patients is the earlier commencement of chemotherapy which has been shown to increase survival from 9 to 24 months and the potential to downstage the disease (93,94).
Numerous studies have confirmed the significant improvement in the quality of life in stage IV CRC patients who were successfully stented for their malignant obstruction. A randomized controlled trial performed by Young et al. showed that patients stenting in patients with obstructed stage IV disease was associated with better quality of life outcomes when compared to baseline at 1 week (58% vs. 27%), and at 12 months (P=0.001 and P=0.01), without worse clinical outcomes in terms of 30-day mortality and median overall survival (95). This concurs with non-randomised studies which have shown improved overall quality of life, as well as life relating to gastrointestinal symptoms in patients who underwent stenting instead of emergency surgical decompression (96).
Way ahead
The indication of SEMS can be expanded to include benign conditions but this remains debatable for there is a need to reconcile the risk of stent related complications (97). In the management of benign strictures, the use of biodegradable stents have been attempted. The case series from the Czech Republic included three patients with Crohn’s disease where balloon dilatation of the stenosis followed by biodegradable stent placement showed favorable results (i.e., degradable of stent within 4 months), with no stent migration or major complications (98).
In addition, alternative management of anastomotic leaks after colorectal surgery, is the novel use of covered SEMS. Reports of success rates ranging from 53.3% to 73.3% have been seen (99). However, increased risk of stent migration (up to 40%) given the used of covered SEMS will have to be undertaken.
Conclusions
Benefits of the use of SEMS as a bridge to surgery should be compared with the potential risk of complications arising from endoscopic colonic stenting. Given ongoing review of its long-term outcomes (i.e., local recurrence and metastatic spread) and safety profile (i.e., stent related adverse events), the official statement from the ESGE and the American Society for Gastrointestinal Endoscopy (ASGE) recommends that (I) the use of colonic SEMS as a bridge to elective surgery is not the standard treatment of symptomatic left-sided malignant colonic obstruction and (II) SEMS placement may be considered as an alternative to emergency surgery in those who have increased risk of post-operative mortality [i.e., American Society of Anaesthesiologists (ASA) Physical Status ≥III and/or >70 years old (34,73)]. Conflicting data are present, and definitive indication requires further evaluation and debate.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Cheynel N, Cortet M, Lepage C, et al. Trends in frequency and management of obstructing colorectal cancers in a well-defined population. Dis Colon Rectum 2007;50:1568-75. [Crossref] [PubMed]
- Tuca A, Guell E, Martinez-Losada E, et al. Malignant bowel obstruction in advanced cancer patients: epidemiology, management, and factors influencing spontaneous resolution. Cancer Manag Res 2012;4:159-69. [Crossref] [PubMed]
- Haraguchi N, Ikeda M, Miyake M, et al. Colonic stenting as a bridge to surgery for obstructive colorectal cancer: advantages and disadvantages. Surg Today 2016;46:1310-7. [Crossref] [PubMed]
- Martinez-Santos C, Lobato RF, Fradejas JM, et al. Self-expandable stent before elective surgery vs. emergency surgery for the treatment of malignant colorectal obstructions: comparison of primary anastomosis and morbidity rates. Dis Colon Rectum 2002;45:401-6. [Crossref] [PubMed]
- Chen HS, Sheen-Chen SM. Obstruction and perforation in colorectal adenocarcinoma: an analysis of prognosis and current trends. Surgery 2000;127:370-6. [Crossref] [PubMed]
- Tan KK, Sim R. Surgery for obstructed colorectal malignancy in an Asian population: predictors of morbidity and comparison between left- and right-sided cancers. J Gastrointest Surg 2010;14:295-302. [Crossref] [PubMed]
- Tekkis PP, Kinsman R, Thompson MR, et al. The Association of Coloproctology of Great Britain and Ireland study of large bowel obstruction caused by colorectal cancer. Ann Surg 2004;240:76-81. [Crossref] [PubMed]
- Tejero E, Mainar A, Fernández L, et al. New procedure for the treatment of colorectal neoplastic obstructions. Dis Colon Rectum 1994;37:1158-9. [Crossref] [PubMed]
- Kaplan J, Strongin A, Adler DG, et al. Enteral stents for the management of malignant colorectal obstruction. World J Gastroenterol 2014;20:13239-45. [Crossref] [PubMed]
- Alcantara M, Serra-Aracil X, Falco J, et al. Prospective, controlled, randomized study of intraoperative colonic lavage versus stent placement in obstructive left-sided colonic cancer. World J Surg 2011;35:1904-10. [Crossref] [PubMed]
- Arezzo A, Balague C, Targarona E, et al. Colonic stenting as a bridge to surgery versus emergency surgery for malignant colonic obstruction: results of a multicentre randomised controlled trial (ESCO trial). Surg Endosc 2017;31:3297-305. [Crossref] [PubMed]
- Cheung HY, Chung CC, Tsang WW, et al. Endolaparoscopic approach vs conventional open surgery in the treatment of obstructing left-sided colon cancer: a randomized controlled trial. Arch Surg 2009;144:1127-32. [Crossref] [PubMed]
- Ghazal AH, El-Shazly WG, Bessa SS, et al. Colonic endolumenal stenting devices and elective surgery versus emergency subtotal/total colectomy in the management of malignant obstructed left colon carcinoma. J Gastrointest Surg 2013;17:1123-9. [Crossref] [PubMed]
- Ho KS, Quah HM, Lim JF, et al. Endoscopic stenting and elective surgery versus emergency surgery for left-sided malignant colonic obstruction: a prospective randomized trial. Int J Colorectal Dis 2012;27:355-62. [Crossref] [PubMed]
- Pirlet IA, Slim K, Kwiatkowski F, et al. Emergency preoperative stenting versus surgery for acute left-sided malignant colonic obstruction: a multicenter randomized controlled trial. Surg Endosc 2011;25:1814-21. [Crossref] [PubMed]
- van Hooft JE, Bemelman WA, Oldenburg B, et al. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol 2011;12:344-52. [Crossref] [PubMed]
- Fiori E, Lamazza A, De Cesare A, et al. Palliative management of malignant rectosigmoidal obstruction. Colostomy vs. endoscopic stenting. A randomized prospective trial. Anticancer Res 2004;24:265-8. [PubMed]
- Abstracts of the Tripartite 2005 Colorectal Meeting, Dublin, Ireland, 5-7 July 2005. Colorectal Dis 2005;7 Suppl 1:1-143. [PubMed]
- Baer C, Menon R, Bastawrous S, et al. Emergency Presentations of Colorectal Cancer. Surg Clin North Am 2017;97:529-45. [Crossref] [PubMed]
- Hennekinne-Mucci S, Tuech JJ, Brehant O, et al. Emergency subtotal/total colectomy in the management of obstructed left colon carcinoma. Int J Colorectal Dis 2006;21:538-41. [Crossref] [PubMed]
- Feo L, Polcino M, Nash GM. Resection of the Primary Tumor in Stage IV Colorectal Cancer: When Is It Necessary? Surg Clin North Am 2017;97:657-69. [Crossref] [PubMed]
- Repici A, Fregonese D, Costamagna G, et al. Ultraflex precision colonic stent placement for palliation of malignant colonic obstruction: a prospective multicenter study. Gastrointest Endosc 2007;66:920-7. [Crossref] [PubMed]
- Repici A, De Caro G, Luigiano C, et al. WallFlex colonic stent placement for management of malignant colonic obstruction: a prospective study at two centers. Gastrointest Endosc 2008;67:77-84. [Crossref] [PubMed]
- Small AJ, Baron TH. Comparison of Wallstent and Ultraflex stents for palliation of malignant left-sided colon obstruction: a retrospective, case-matched analysis. Gastrointest Endosc 2008;67:478-88. [Crossref] [PubMed]
- Fregonese D, Naspetti R, Ferrer S, et al. Ultraflex precision colonic stent placement as a bridge to surgery in patients with malignant colon obstruction. Gastrointest Endosc 2008;67:68-73. [Crossref] [PubMed]
- Kim JH, Song HY, Li YD, et al. Dual-design expandable colorectal stent for malignant colorectal obstruction: comparison of flared ends and bent ends. AJR Am J Roentgenol 2009;193:248-54. [Crossref] [PubMed]
- Repici A, de Paula Pessoa Ferreira D. Expandable metal stents for malignant colorectal strictures. Gastrointest Endosc Clin N Am 2011;21:511-33. ix. [Crossref] [PubMed]
- Kim EJ, Kim YJ. Stents for colorectal obstruction: Past, present, and future. World J Gastroenterol 2016;22:842-52. [Crossref] [PubMed]
- Zhang Y, Shi J, Shi B, et al. Comparison of efficacy between uncovered and covered self-expanding metallic stents in malignant large bowel obstruction: a systematic review and meta-analysis. Colorectal Dis 2012;14:e367-74. [Crossref] [PubMed]
- Yang Z, Wu Q, Wang F, et al. A systematic review and meta-analysis of randomized trials and prospective studies comparing covered and bare self-expandable metal stents for the treatment of malignant obstruction in the digestive tract. Int J Med Sci 2013;10:825-35. [Crossref] [PubMed]
- Dabizzi E, Arcidiacono PG. Update on Enteral Stents. Curr Treat Options Gastroenterol 2016;14:178-84. [Crossref] [PubMed]
- Vitale MA, Villotti G, d'Alba L, et al. Preoperative colonoscopy after self-expandable metallic stent placement in patients with acute neoplastic colon obstruction. Gastrointest Endosc 2006;63:814-9. [Crossref] [PubMed]
- van Hooft JE, van Halsema EE, Vanbiervliet G, et al. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2014;46:990-1053. [Crossref] [PubMed]
- Fugazza A, Galtieri PA, Repici A. Using stents in the management of malignant bowel obstruction: the current situation and future progress. Expert Rev Gastroenterol Hepatol 2017;11:633-41. [Crossref] [PubMed]
- Saito S, Yoshida S, Isayama H, et al. A prospective multicenter study on self-expandable metallic stents as a bridge to surgery for malignant colorectal obstruction in Japan: efficacy and safety in 312 patients. Surg Endosc 2016;30:3976-86. [Crossref] [PubMed]
- Cirocchi R, Farinella E, Trastulli S, et al. Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: a systematic review and meta-analysis. Surg Oncol 2013;22:14-21. [Crossref] [PubMed]
- Manes G, de Bellis M, Fuccio L, et al. Endoscopic palliation in patients with incurable malignant colorectal obstruction by means of self-expanding metal stent: analysis of results and predictors of outcomes in a large multicenter series. Arch Surg 2011;146:1157-62. [Crossref] [PubMed]
- Little MW, Oakley T, Briggs JH, et al. Technical and Clinical Outcomes Following Colonic Stenting: A Seven-Year Analysis of 268 Procedures. Cardiovasc Intervent Radiol 2016;39:1471-8. [Crossref] [PubMed]
- Lim TZ, Chan D, Tan KK. Patients who failed endoscopic stenting for left-sided malignant colorectal obstruction suffered the worst outcomes. Int J Colorectal Dis 2014;29:1267-73. [Crossref] [PubMed]
- Baron TH, Wong Kee Song LM, Repici A. Role of self-expandable stents for patients with colon cancer (with videos). Gastrointest Endosc 2012;75:653-62. [Crossref] [PubMed]
- Song HY, Kim JH, Shin JH, et al. A dual-design expandable colorectal stent for malignant colorectal obstruction: results of a multicenter study. Endoscopy 2007;39:448-54. [Crossref] [PubMed]
- Ngu J, Lieske B, Chan KH, et al. Caecal pneumatosis is not an absolute contraindication for endoluminal stenting in patients with acute malignant large bowel obstruction. ANZ J Surg 2014;84:772-5. [Crossref] [PubMed]
- Kim JH, Ku YS, Jeon TJ, et al. The efficacy of self-expanding metal stents for malignant colorectal obstruction by noncolonic malignancy with peritoneal carcinomatosis. Dis Colon Rectum 2013;56:1228-32. [Crossref] [PubMed]
- Trompetas V, Saunders M, Gossage J, et al. Shortcomings in colonic stenting to palliate large bowel obstruction from extracolonic malignancies. Int J Colorectal Dis 2010;25:851-4. [Crossref] [PubMed]
- Shin SJ, Kim TI, Kim BC, et al. Clinical application of self-expandable metallic stent for treatment of colorectal obstruction caused by extrinsic invasive tumors. Dis Colon Rectum 2008;51:578-83. [Crossref] [PubMed]
- Caceres A, Zhou Q, Iasonos A, et al. Colorectal stents for palliation of large-bowel obstructions in recurrent gynecologic cancer: an updated series. Gynecol Oncol 2008;108:482-5. [Crossref] [PubMed]
- Miyayama S, Matsui O, Kifune K, et al. Malignant colonic obstruction due to extrinsic tumor: palliative treatment with a self-expanding nitinol stent. AJR Am J Roentgenol 2000;175:1631-7. [Crossref] [PubMed]
- Kim JH, Song HY, Park JH, et al. Metallic stent placement in the palliative treatment of malignant colonic obstructions: primary colonic versus extracolonic malignancies. J Vasc Interv Radiol 2011;22:1727-32. [Crossref] [PubMed]
- Keswani RN, Azar RR, Edmundowicz SA, et al. Stenting for malignant colonic obstruction: a comparison of efficacy and complications in colonic versus extracolonic malignancy. Gastrointest Endosc 2009;69:675-80. [Crossref] [PubMed]
- Luigiano C, Ferrara F, Fabbri C, et al. Through-the-scope large diameter self-expanding metal stent placement as a safe and effective technique for palliation of malignant colorectal obstruction: a single center experience with a long-term follow-up. Scand J Gastroenterol 2011;46:591-6. [Crossref] [PubMed]
- Bonin EA, Baron TH. Update on the indications and use of colonic stents. Curr Gastroenterol Rep 2010;12:374-82. [Crossref] [PubMed]
- Small AJ, Coelho-Prabhu N, Baron TH. Endoscopic placement of self-expandable metal stents for malignant colonic obstruction: long-term outcomes and complication factors. Gastrointest Endosc 2010;71:560-72. [Crossref] [PubMed]
- Ansaloni L, Andersson RE, Bazzoli F, et al. Guidelenines in the management of obstructing cancer of the left colon: consensus conference of the world society of emergency surgery (WSES) and peritoneum and surgery (PnS) society. World J Emerg Surg 2010;5:29. [Crossref] [PubMed]
- Williams D, Law R, Pullyblank AM. Colorectal stenting in malignant large bowel obstruction: the learning curve. Int J Surg Oncol 2011;2011:917848. [Crossref] [PubMed]
- Sagar J. Colorectal stents for the management of malignant colonic obstructions. Cochrane Database Syst Rev 2011.CD007378. [PubMed]
- Tan CJ, Dasari BV, Gardiner K. Systematic review and meta-analysis of randomized clinical trials of self-expanding metallic stents as a bridge to surgery versus emergency surgery for malignant left-sided large bowel obstruction. Br J Surg 2012;99:469-76. [Crossref] [PubMed]
- Ng SS, Lee JF, Yiu RY, et al. Emergency laparoscopic-assisted versus open right hemicolectomy for obstructing right-sided colonic carcinoma: a comparative study of short-term clinical outcomes. World J Surg 2008;32:454-8. [Crossref] [PubMed]
- Rho SY, Bae SU, Baek SJ, et al. Feasibility and safety of laparoscopic resection following stent insertion for obstructing left-sided colon cancer. J Korean Surg Soc 2013;85:290-5. [Crossref] [PubMed]
- Sebastian S, Johnston S, Geoghegan T, et al. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol 2004;99:2051-7. [Crossref] [PubMed]
- Brehant O, Fuks D, Bartoli E, et al. Elective (planned) colectomy in patients with colorectal obstruction after placement of a self-expanding metallic stent as a bridge to surgery: the results of a prospective study. Colorectal Dis 2009;11:178-83. [Crossref] [PubMed]
- Huang X, Lv B, Zhang S, et al. Preoperative colonic stents versus emergency surgery for acute left-sided malignant colonic obstruction: a meta-analysis. J Gastrointest Surg 2014;18:584-91. [Crossref] [PubMed]
- Park SJ, Lee KY, Kwon SH, et al. Stenting as a Bridge to Surgery for Obstructive Colon Cancer: Does It Have Surgical Merit or Oncologic Demerit? Ann Surg Oncol 2016;23:842-8. [Crossref] [PubMed]
- Allievi N, Ceresoli M, Fugazzola P, et al. Endoscopic Stenting as Bridge to Surgery versus Emergency Resection for Left-Sided Malignant Colorectal Obstruction: An Updated Meta-Analysis. Int J Surg Oncol 2017;2017:2863272. [Crossref] [PubMed]
- Arezzo A, Passera R, Lo Secco G, et al. Stent as bridge to surgery for left-sided malignant colonic obstruction reduces adverse events and stoma rate compared with emergency surgery: results of a systematic review and meta-analysis of randomized controlled trials. Gastrointest Endosc 2017;86:416-26. [Crossref] [PubMed]
- Khot UP, Lang AW, Murali K, et al. Systematic review of the efficacy and safety of colorectal stents. Br J Surg 2002;89:1096-102. [Crossref] [PubMed]
- Han YM, Lee JM, Lee TH. Delayed colon perforation after palliative treatment for rectal carcinoma with bare rectal stent: a case report. Korean J Radiol 2000;1:169-71. [Crossref] [PubMed]
- Baron TH. Colonic stenting: technique, technology, and outcomes for malignant and benign disease. Gastrointest Endosc Clin N Am 2005;15:757-71. [Crossref] [PubMed]
- Suh JP, Kim SW, Cho YK, et al. Effectiveness of stent placement for palliative treatment in malignant colorectal obstruction and predictive factors for stent occlusion. Surg Endosc 2010;24:400-6. [Crossref] [PubMed]
- Branger F, Thibaudeau E, Mucci-Hennekinne S, et al. Management of acute malignant large-bowel obstruction with self-expanding metal stent. Int J Colorectal Dis 2010;25:1481-5. [Crossref] [PubMed]
- Lee HJ, Hong SP, Cheon JH, et al. Long-term outcome of palliative therapy for malignant colorectal obstruction in patients with unresectable metastatic colorectal cancers: endoscopic stenting versus surgery. Gastrointest Endosc 2011;73:535-42. [Crossref] [PubMed]
- Li G, Chen Y, Hu J, et al. A 5-fluorouracil-loaded polydioxanone weft-knitted stent for the treatment of colorectal cancer. Biomaterials 2013;34:9451-61. [Crossref] [PubMed]
- Sabbagh C, Browet F, Diouf M, et al. Is stenting as "a bridge to surgery" an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction? A comparative study with a propensity score analysis. Ann Surg 2013;258:107-15. [Crossref] [PubMed]
- Gorissen KJ, Tuynman JB, Fryer E, et al. Local recurrence after stenting for obstructing left-sided colonic cancer. Br J Surg 2013;100:1805-9. [Crossref] [PubMed]
- Choi JM, Lee C, Han YM, et al. Long-term oncologic outcomes of endoscopic stenting as a bridge to surgery for malignant colonic obstruction: comparison with emergency surgery. Surg Endosc 2014;28:2649-55. [Crossref] [PubMed]
- Thorlacius H. Tumour cell dissemination following endoscopic stent insertion (Br J Surg 2007; 94: 1151-1154). Br J Surg 2008;95:127-8; author reply 8. [Crossref] [PubMed]
- Sloothaak DA, van den Berg MW, Dijkgraaf MG, et al. Oncological outcome of malignant colonic obstruction in the Dutch Stent-In 2 trial. Br J Surg 2014;101:1751-7. [Crossref] [PubMed]
- Zhang Y, Shi J, Shi B, et al. Self-expanding metallic stent as a bridge to surgery versus emergency surgery for obstructive colorectal cancer: a meta-analysis. Surg Endosc 2012;26:110-9. [Crossref] [PubMed]
- Zhao X, Liu B, Zhao E, et al. The safety and efficiency of surgery with colonic stents in left-sided malignant colonic obstruction: a meta-analysis. Gastroenterol Res Pract 2014;2014:407325. [Crossref] [PubMed]
- Zahid A, Young CJ. How to decide on stent insertion or surgery in colorectal obstruction? World J Gastrointest Surg 2016;8:84-9. [Crossref] [PubMed]
- Liang TW, Sun Y, Wei YC, et al. Palliative treatment of malignant colorectal obstruction caused by advanced malignancy: a self-expanding metallic stent or surgery? A system review and meta-analysis. Surg Today 2014;44:22-33. [Crossref] [PubMed]
- Zhao XD, Cai BB, Cao RS, et al. Palliative treatment for incurable malignant colorectal obstructions: a meta-analysis. World J Gastroenterol 2013;19:5565-74. [Crossref] [PubMed]
- Takahashi H, Okabayashi K, Tsuruta M, et al. Self-Expanding Metallic Stents Versus Surgical Intervention as Palliative Therapy for Obstructive Colorectal Cancer: A Meta-analysis. World J Surg 2015;39:2037-44. [Crossref] [PubMed]
- Kaushik M, Bhullar JS, Bindroo S, et al. Minimally Invasive Management of Complicated Diverticular Disease: Current Status and Review of Literature. Dig Dis Sci 2016;61:663-72. [Crossref] [PubMed]
- Cennamo V, Fuccio L, Mutri V, et al. Does stent placement for advanced colon cancer increase the risk of perforation during bevacizumab-based therapy? Clin Gastroenterol Hepatol 2009;7:1174-6. [Crossref] [PubMed]
- Kemmochi T, Egawa T, Mihara K, et al. Neoadjuvant chemotherapy with capecitabine plus oxaliplatin and bevacizumab for the treatment of patients with resectable metastatic colorectal cancer. Gan To Kagaku Ryoho 2013;40:1629-31. [PubMed]
- Misiakos EP, Karidis NP, Kouraklis G. Current treatment for colorectal liver metastases. World J Gastroenterol 2011;17:4067-75. [Crossref] [PubMed]
- Kleespies A, Fuessl KE, Seeliger H, et al. Determinants of morbidity and survival after elective non-curative resection of stage IV colon and rectal cancer. Int J Colorectal Dis 2009;24:1097-109. [Crossref] [PubMed]
- Giuliante F, Ardito F, Vellone M, et al. Role of the surgeon as a variable in long-term survival after liver resection for colorectal metastases. J Surg Oncol 2009;100:538-45. [Crossref] [PubMed]
- Wei AC, Greig PD, Grant D, et al. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol 2006;13:668-76. [Crossref] [PubMed]
- Dienstmann R, Salazar R, Tabernero J. Personalizing colon cancer adjuvant therapy: selecting optimal treatments for individual patients. J Clin Oncol 2015;33:1787-96. [Crossref] [PubMed]
- Liberman H, Adams DR, Blatchford GJ, et al. Clinical use of the self-expanding metallic stent in the management of colorectal cancer. Am J Surg 2000;180:407-11; discussion 412. [Crossref] [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [Crossref] [PubMed]
- Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 2009;27:663-71. [Crossref] [PubMed]
- Young CJ, De-Loyde KJ, Young JM, et al. Improving Quality of Life for People with Incurable Large-Bowel Obstruction: Randomized Control Trial of Colonic Stent Insertion. Dis Colon Rectum 2015;58:838-49. [Crossref] [PubMed]
- Nagula S, Ishill N, Nash C, et al. Quality of life and symptom control after stent placement or surgical palliation of malignant colorectal obstruction. J Am Coll Surg 2010;210:45-53. [Crossref] [PubMed]
- Li Y, Wu JH, Meng Y, et al. New devices and techniques for endoscopic closure of gastrointestinal perforations. World J Gastroenterol 2016;22:7453-62. [Crossref] [PubMed]
- ASGE Technology Committee, Tokar JL, Banerjee S, et al. Drug-eluting/biodegradable stents. Gastrointest Endosc 2011;74:954-8. [Crossref]
- Arezzo A, Bini R, Lo Secco G, et al. The role of stents in the management of colorectal complications: a systematic review. Surg Endosc 2017;31:2720-30. [Crossref] [PubMed]