
Adaptive radiation therapy for cervical esophageal cancer: dosimetric and volumetric analysis
Introduction
Cervical esophageal cancer (CEC) is rarely seen and accounts for 2–10% of all esophageal cancers (1,2). Treatment methods and the results of CEC differs from thoracic or abdominal esophageal cancers. RT is the primary treatment modality for CEC (3). Studies are suggesting that high dose radiation should be given in the treatment of CEC similar to the head and neck (HN) cancers (4).
The use of modern RT techniques allows treatment of CEC with low toxicity (5). Intensity-modulated radiotherapy (IMRT) has become the standard technique for the treatment of many cancers (6). One of the optimal techniques for CEC is typically IMRT. Increased of coverage, conformality and decreased dose to organs at risk (OARs) such as medulla spinalis (MS), brainstem, oral cavity, and parotid glands can be obtained with IMRT plans (7). When the image-guided RT (IGRT) is added to the IMRT method; organ movement and set-up changes can be easily detected (8).
Helical tomotherapy (HT) (Accuray Inc., Madison, WI, USA) has daily megavoltage computed tomography (MVCT) which is used for pretreatment patient positioning to decrease setup errors and see anatomical changes (9). But there may be differences between the planning dose and the verification dose, depending on the patient’s postures and anatomical changes during treatment and it may lead to under dosage or overdosage in target and OARs (10,11). The IG-IMRT technique does not allow dose and contour change while the treatment is going on. So the adaptive radiotherapy (ART) technique started to be used. Owing to the ART, daily dosing can be calculated considering daily anatomical changes and the comparison of the planning and verification dose can be done. ART is an important approach to make the correction of the daily tumor and OAR’s variations through streamlined online or offline modification of original planning volumes and plans (12). The offline ART modification is widely used and is based on the principle of new CT extraction during treatment. These new CT images are used for recontouring and replanning (13).
Volumetric and dosimetric changes may occur in CEC patients during 6–7 weeks of RT treatment. The aim of the study is to evaluate the volumetric and dosimetric changes in target and OARs in CEC patients by using ART technique.
Methods
Patient selection
Seven patients who had radical RT in HT device between February 2015 and January 2018 for the treatment of CEC and needed ART planning because of the tumor shrinkage or weight lost causing in volumetric and dosimetric changes in the RT field were selected for the study. Our study was approved by the Erzurum Regional Education and Research Hospital Ethics Committee of Clinical Trials, Turkey (Erzurum BEAH KAEK 2019/04-37). Study was a retrospective review so subject informed consent was not obtained.
Patient characteristics are shown in Table 1.
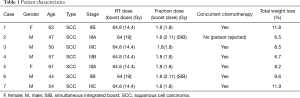
Full table
Simulation, delineation and radiotherapy planning
Patients were immobilized with a supine position with both arms by their sides and using a thermoplastic IMRT mask covering the HN and shoulders (type-S thermoplastic-based system CIVCO, Civco Medical Solutions, Kalona, IA, USA). CT images were taken with 3-mm slice thickness throughout the entire HN and thorax regions. Second and third CT scans were performed with a new thermoplastic mask used same baseplate and head support during the course of treatment at 3rd (CT2) and 5th (CT3) weeks for all patients. The weight of each patient was recorded before CT1, CT2, and CT3. CT images were transmitted to the contouring workstation through Digital Imaging and Communications in Medicine (DICOM). The OARs (brain, brainstem, MS, parotid and submandibular glands, oral cavity) were delineated first at the Focal Sim (ver.4.80) contouring workstation. The gross tumor volume (GTV) was contoured according to the findings at the staging tests (upper gastrointestinal endoscopy, diagnostic tomography, PET-CT). A 3–5 cm craniocaudal margin was given for clinical target volume (CTV). Also, the bilateral cervical lymphatic regions, supraclavicular and upper mediastinal lymph nodes are included to the CTV. The margin for planning target volume (PTV) was 5 mm. After all volumes were constructed the CT images and structures were transferred to tomotherapy planning system (TPS) (Accuray Inc., Madison, USA).
Twenty-one IMRT plans were performed in initial CT (CT1), 3rd and 5th weeks CT (CT2, CT3) in TPS (Accuray Inc.). For all plans, a field width of 2.5 cm, a pitch of 0.287 and a modulation factor of 2.0 was used during optimization and dose calculation. Total 64.8 Gy doses were defined for cases 1, 3, 4, 5 and 7. Total 60 Gy doses were defined for cases 2 and 6. A daily dose of 1.8 Gy was given to gross PTV. Simultaneous integrated boost (SIB) technique was adopted in cases 2 and 6. For this case 64 Gy total dose was planned and daily doses of PTV and PTV boost was 1.8 and 2.11 Gy, respectively.
Adaptive planning and analysis of the recalculated dose distributions
All patients have been treated using daily MV image guidance in HT. The ART software (Accuray Inc., Madison, WI, USA) module allows dose to be recalculated based on the MVCT. Daily MVCT images were used for the offline ART modification to determine how the daily positioning and anatomical changes of each fraction affect the target coverage and OAR’s dose difference. In this system, the dose is calculated according to daily anatomy and DVH is obtained. In this study, an off-line ART plan was performed pre-CT2 and CT3. The DVH of the treatment plan and daily DVH are compared and evaluated. Volumetric and dosimetric differences in PTV and OAR’s were analyzed.
Statistical analysis
Percentage differences were calculated by volume comparison (PTV, PTV boost, right parotid, and left parotid) between CT1, CT2, and CT3. Dosimetric comparison of the PTV’s D95, D50 and Dmax between CT1 and ART before verification of CT2 and ART before verification of CT3 values were performed. The analyzed variables were D95, Dmean, Dmin, Dmax for PTV and Dmean and Dmax for OARs. Wilcoxon Singed Ranks Test was used to compare the PTV and OAR’s doses between CT1, CT2, and CT3. A value of P<0.05 was considered significant. All statistics were calculated by using SPSS 18.0 statistical software (SPSS Inc., Chicago, USA).
Results
The patients who will benefit most from replanning are decided with MVCT imaging analysis and daily ART’s DVH analysis in our clinic. Tumor shrinkage and weight loss during treatment are the most important factors in ART decision. In the present study, we investigate the volumetric and dosimetric changes in CEC, which is a rare type of esophageal cancer by using ART technique. Volumetric and dosimetric [V(cc), D95%, Dmean, D50, Dmin, Dmax] changes of target and OARs (parotid glands, MS, brainstem and oral cavity) values were evaluated.
The volumetric changes between first plans and recalculated plans
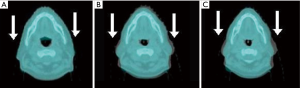
The average weight loss of the patients during the treatment period was 9.03%. Statistical analysis revealed a significant decrease in PTV, PTV boost, left parotid and right parotid volume values (P<0.05). The major volume changes were 4.74% for PTV, 15.93% for PTV boost, 26.82% for right parotid and 26.64% for left parotid. Volumetrically, the CT1-CT3 percent differences values were greater than CT1-CT2. The details of the volumetric comparisons are given in Table 2. The volumetric changes in the irradiation field during the treatment course for case 1 showed in Figure 1.
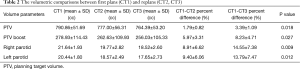
Full table
Analysis of ART
Using ART planning DVH was evaluated with first planning PTV values (CT1) and PTV values of pre-CT2 and CT3 shown in Table 3. The decrease of D95 (CT1-CT3) and the increase of Dmax values (CT1-CT2 and CT1-CT3) were statistically significant (P<0.05).

Full table
The dosimetric changes between first plans (CT1) and recalculated plans (CT2 and CT3)
Impact of anatomical changes in PTV and OARs (brainstem, MS, parotid glands, oral cavity) on dosimetric outcome was evaluated. Table 4 showed the dose difference of PTV and OARs between CT1, CT2, and CT3. When the dose differences of CT3-CT1 was evaluated, the maximum decrease of the left parotid was 19.01% and right parotid was 16.04%. The mean dose reductions of left and right parotid were 2.48 and 2.49 Gy, respectively. These decreases were significant for both parotid glands (P<0.05). No significant correlation was observed between CT1-CT2 and CT1-CT3 summation plan values of PTV (D95, Dmean, Dmin, and Dmax) and Dmax values of MS, brainstem and oral cavity.

Full table
Discussion
Patients who are receiving RT and concurrent chemotherapy may experience weight loss due to dysphagia, odynophagia, mucositis, taste disorders, nausea, vomiting, diarrhea, increased catabolism and depression. In the present study, we found the average weight loss of the CEC patients during the treatment period was 9.03%. In addition, a mean volume reduction of 3.76% and 8.19% was observed for PTV and PTV boost during the 5–7 weeks treatment period, respectively. In the literature, volumetric and dosimetric changes during treatment were examined especially for HN cancers. In the study of Bando et al. there was a 7% reduction in the weight of the patients during the first 3 weeks of the treatment, a 28% reduction in the target volume and an 11% reduction in the neck volume in HN RT (14). In Yip and colleagues’ study, there was a decrease of 4.7% in PTV1 volume and 11.5% in PTV2 volume (15).
Weight loss, the shrinkage, and deformation of the target and OAR are expected during RT (16). Several studies have documented significant volumetric change in parotids during RT for HN cancers. In the study of Ho et al., a weight loss of 6.5 kg was found in the treatment process. They found that ipsilateral and contralateral parotid lesions showed a mean decrease of 29.7% and 28.4%, respectively (17). In the study of Yip et al., a decrease of 10.4% in the single parotid volume and 12.1% decrease in total parotid volume were determined (15). In another study, mean parotid gland volume reduction on the 3rd week of the treatment, at the end of the treatment and second month after treatment were 20%, 26.9%, and 27.2%, respectively (18). In a similar study, the parotid glands showed a progressive mean volume reduction of 22% at 5th day CT and 30% at 25th day CT (19). In our analysis, we found that right and left parotid volumes showed a mean decrease of 14.40% and 13.80% respectively.
The change of the patients’ external contour causes different dose distributions to the target and critical structures. Anatomical changes during the treatment can result in a high dose to the OARs than expected (20). Our findings consistent that the mean dose reductions of left and right parotids were 2.48 and 2.49 Gy, respectively. The HN study of O’Daniel et al., showed that parotid gland doses were found 5–7 Gy higher in 45% of patients (21). Robar et al. found in their study that the change in mean dose to the parotids was 2.6% (22). Schwartz et al. noted mean parotid dose sparing by 3.9% and 3.8% in contralateral parotid and by 2.8% and 9% in ipsilateral parotid with single and two ART planning, respectively (23). In the study of Wang et al. found that there was a 1% decrease in the mean dose of left parotid and 1.3% decrease in right parotid and Ahn et al. found a 24% reduction in parotid volume with ART and a 22% increase in parotid conservation with re-planning (24,25). Castadot et al. reported that reduction in mean parotid dose after 1, 2 and 6 weeks replanning by 3%, 5%, and 6%, respectively (20).
In our study, when we compare the first CT plan and the replanning data, no significant correlation was observed between CT1-CT2 and CT1-CT3 summation plan values of MS, brainstem and oral cavity. Similarly in the study of Beltran et al., showed that no significant dose changes were found in the OARs (oral cavity, brainstem, MS, optic chiasm, optic nerves) (19).
In the treatment process, the target and the volumetric changes in the critical organs require a change in the treatment planning. ART consists of evaluating the dose distribution during treatment and replanning if necessary, depending on the patient’s anatomical changes (26). With ART, daily changes in the treatment plan can be observed. In this study, we found a significant dosimetric change of the targets between the CT1 planning values to CT2-CT3 ART before verification values.
The limitation of the present study is containing a small number of patients, and thus further studies with a larger patient population are needed to consolidate the importance of ART planning in CEC patients.
Conclusions
The ART technique is feasible and should be considered in the RT of CEC patients who are at risk of volumetric changes during the treatment due to the weight loss like any other HN cancer patient. Our volumetric and dosimetric results showed that using ART technique was beneficial to ensure adequate doses to the target volumes and safe doses to the OAR for the patients who need replanning during RT in a rare form of esophageal cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Our study was approved by the Erzurum Regional Education and Research Hospital Ethics Committee of Clinical Trials (Erzurum BEAH KAEK 2019/04-37). Study was a retrospective review so subject informed consent was not obtained.
References
- Lee DJ, Harris A, Gillette A, et al. Carcinoma of the cervical esophagus: diagnosis, management, and results. South Med J 1984;77:1365-7. [Crossref] [PubMed]
- Hoeben A, Polak J, Van De Voorde L, et al. Cervical esophageal cancer: a gap in cancer knowledge. Ann Oncol 2016;27:1664-74. [Crossref] [PubMed]
- Fenkell L, Kaminsky I, Breen S, et al. Dosimetric comparison of IMRT vs. 3D conformal radiotherapy in the treatment of cancer of the cervical esophagus. Radiother Oncol 2008;89:287-91. [Crossref] [PubMed]
- Yamada K, Murakami M, Okamoto Y, et al. Treatment results of radiotherapy for carcinoma of the cervical esophagus. Acta Oncol 2006;45:1120-5. [Crossref] [PubMed]
- Zhao L, Zhou Y, Mu Y, et al. Patterns of failure and clinical outcomes of definitive radiotherapy for cervical esophageal cancer. Oncotarget 2017;8:21852-60. [PubMed]
- Lee N, Xia P, Fischbein NJ, et al. Intensity-modulated radiation therapy for head-and-neck cancer: the UCSF experience focusing on target volume delineation. Int J Radiat Oncol Biol Phys 2003;57:49-60. [Crossref] [PubMed]
- Saba NF, El-Rayes BF. editors. Esophageal cancer: Prevention, Diagnosis and Therapy. Cham: Springer International Publishing, 2015.
- Yoo DS, Wong TZ, Brizel DM. The role of adaptive and functional imaging modalities in radiation therapy: Approach and application from a radiation oncology perspective. Semin Ultrasound CT MR 2010;31:444-61. [Crossref] [PubMed]
- Langen KM, Willoughby TR, Meeks SL, et al. Observations on real-time prostate gland motion using electromagnetic tracking. Int J Radiat Oncol Biol Phys 2008;71:1084-90. [Crossref] [PubMed]
- Yan D, Lockman D, Martinez A, et al. Computed tomography guided management of interfractional patient variation. Semin Radiat Oncol 2005;15:168-79. [Crossref] [PubMed]
- Hansen EK, Bucci MK, Quivey JM, et al. Repeat CT imaging and replanning during the course of IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2006;64:355-62. [Crossref] [PubMed]
- Brouwer CL, Steenbakkers RJ, Langendijk JA, et al. Identifying patients who may benefit from adaptive radiotherapy: does the literature on anatomic and dosimetric changes in head and neck organs at risk during radiotherapy provide information to help? Radiother Oncol 2015;115:285-94. [Crossref] [PubMed]
- Nuver TT, Hoogeman MS, Remeijer P, et al. An adaptive off-line procedure for radiotherapy of prostate cancer. Int J Radiat Oncol Biol Phys 2007;67:1559-67. [Crossref] [PubMed]
- Bando R, Ikushima H, Kawanaka T, et al. Changes of tumor and normal structures of the neck during radiation therapy for head and neck cancer requires adaptive strategy. J Med Invest 2013;60:46-51. [Crossref] [PubMed]
- Yip C, Thomas C, Michaelidou A, et al. Co-registration of cone beam CT and planning CT in head and neck IMRT dose estimation: a feasible adaptive radiotherapy strategy. Br J Radiol 2014;87:20130532. [Crossref] [PubMed]
- Broggi S, Fiorino C, Dell'Oca I, et al. A two-variable linear model of parotid shrinkage during IMRT for head and neck cancer. Radiother Oncol 2010;94:206-12. [Crossref] [PubMed]
- Ho KF, Marchant T, Moore C, et al. Monitoring dosimetric impact of weight loss with kilovoltage (kV) cone beam CT (CBCT) during parotid-sparing IMRT and concurrent chemotherapy. Int J Radiat Oncol Biol Phys 2012;82:e375-82. [Crossref] [PubMed]
- Wang ZH, Yan C, Zhang ZY, et al. Radiation-induced volume changes in parotid and submandibular glands in patients with head and neck cancer receiving postoperative radiotherapy: a longitudinal study. Laryngoscope 2009;119:1966-74. [Crossref] [PubMed]
- Beltran M, Ramos M, Rovira JJ, et al. Dose variations in tumor volumes and organs at risk during IMRT for head-and-neck cancer. J Appl Clin Med Phys 2012;13:3723. [Crossref] [PubMed]
- Castadot P, Geets X, Lee JA, et al. Adaptive functional image-guided IMRT in pharyngo-laryngeal squamous cell carcinoma: is the gain in dose distribution worth the effort? Radiother Oncol 2011;101:343-50. [Crossref] [PubMed]
- O'Daniel JC, Garden AS, Schwartz DL. Parotid gland dose in intensity-modulated radiotherapy for head and neck cancer: Is what you plan what you get? Int J Radiat Oncol Biol Phys 2007;69:1290-6. [Crossref] [PubMed]
- Robar JL, Day A, Clancey J, et al. Spatial and dosimetric variability of organs at risk in head and neck intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys 2007;68:1121-30. [Crossref] [PubMed]
- Schwartz DL, Garden AS, Thomas J, et al. Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys 2012;83:986-93. [Crossref] [PubMed]
- Wang W, Yang H, Hu W, et al. Clinical study of the necessity of replanning before the 25th fraction during the course of intensity-modulated radiotherapy for patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2010;77:617-21. [Crossref] [PubMed]
- Ahn PH, Chen CC, Ahn AI, et al. Adaptive planning in intensity-modulated radiation therapy for head and neck cancers: single-institution experience and clinical implications. Int J Radiat Oncol Biol Phys 2011;80:677-85. [Crossref] [PubMed]
- Collet S, Nomikossoff N, Garnier E, et al. Adaptive radiotherapy with tomotherapy: Assessment of Planned Adaptive software to clinical use for head and neck tumor patient with orbital involvement. Physica Medica 2015;31:36. [Crossref]


