
Clinicopathological features and outcomes of fibrolamellar hepatocellular carcinoma
Introduction
Fibrolamellar hepatocellular carcinoma (FLHCC) is a rare primary malignant neoplasm of the liver accounting for 0.8% to 16% of all hepatocellular malignancies (1). Histologically, FLHCC consists of large polygonal tumor cells with eosinophilic granular cytoplasm, large vesicular nuclei, and prominent nucleoli with an immunohistochemical profile similar to that of hepatocellular carcinoma, such as positive staining for hepatocyte paraffin 1 (HepPar1), glypican-3 (GPC3), arginase-1, polyclonal carcinoembryonic antigen (pCEA), and CD10 positivity. However, immunostaining for CD68 and epithelial membrane antigen (EMA) are specific for FLHCC (2-4). The tumor has a striking intra-tumoral fibrosis, classically arranged in parallel or lamellar bands (5). Given the rarity of the disease, most of our knowledge on this cancer is obtained from small case series. Unlike conventional hepatocellular carcinoma, FLHCC has not been linked with risk factors such as viral hepatitis, chronic liver disease, alcohol, or estrogen use. The FLHCC is known to occur more frequently in younger population with a wide variation in reported median age, ranging from 20 to 32 years. The clinical symptoms of FLHCC are usually nonspecific and may include nausea, abdominal discomfort or fullness, weight loss, and/or night sweats (6). Although disease stage and resectability are considered to be important prognostic factors, retrospective studies have reported discrepant findings (6-11). There is some debate as to whether FLHCC has a better prognosis than conventional hepatocellular carcinoma (HCC) as suggested by the United States Surveillance, Epidemiology, and End Results (SEER) database analysis (12). Similar long-term survival rates with hepatocellular carcinoma and FLHCC have been reported in other studies (13,14). Recent studies have shown that FLHCC is associated with the presence of DNAJB1-PRKACA fusion mutations (9). Though surgical resection is the mainstay of therapy, limited data exist on the effective therapies for metastatic and recurrent disease. We retrospectively evaluated 42 cases of FLHCC treated at Mayo Clinic between January 1990 and December 2017 in terms of clinicopathological features, treatment, recurrence pattern and survival outcomes with various therapies.
Methods
Study design and study population
The cancer registry of Mayo Clinic was searched for patients with a diagnosis of FLHCC who were treated between 1990 and 2017 at the three sites of Mayo Clinic (Rochester, MN; Scottsdale, AZ; and Jacksonville, FL). Patients with histologically confirmed diagnosis of FLHCC were included in the analysis. Patients (n=3) were excluded from the study if the initial staging could not be accurately determined from the chart review or if the patient had incomplete treatment record.
Data were collected retrospectively by chart review on demographics, staging, pathology, treatment received including surgery and systemic therapy, recurrence pattern and survival. The primary objectives of the analysis were to evaluate: (I) clinicopathological characteristics in relation to outcome and (II) response to various therapies. The disease stage was determined for each patient according to the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC), tumor, nodal and metastasis (TNM) staging system of primary liver cancer, 7th edition (in 2010). The study was approved by the Institutional Review Board of Mayo Clinic.
Statistical analysis
We used descriptive statistics to provide a summary of the data. Absolute and relative frequencies (percentages) were used to describe categorical data. Continuous data were presented as median, minimum to maximum range. Non-normally distributed continuous variables were expressed as median and range. One-way analysis of variance was used to compare continuous variables and Chi-square tests were used to compare categorical variables. Overall Survival (OS) was estimated by the Kaplan-Meier method and compared within groups using log-rank tests. The multivariable analysis was done using Cox regression modelling. Statistical analysis was performed using SPSS statistical software (version 24.0; SPSS, Inc., Chicago, IL, USA). Statistical significance was determined as P<0.05 with two-sided test.
Results
Baseline characteristics
The patient population (n=42) consisted of 17 males and 25 females, with a median age at diagnosis of 22 years (range, 15 to 39). The median follow-up period was 38 months. The tumor involved the right lobe of liver in 23 patients and the left lobe in 19 patients. Three patients had multifocal disease. None had underlying cirrhosis or chronic hepatitis. Distant metastasis at diagnosis was present in 14 patients with peritoneum, lymph nodes and lungs being the most common sites of metastases. The baseline characteristics of patients and stage (AJCC 7th edition) distribution are summarized in Table 1. Lympho-vascular and peri-neural invasion was present in 9 (21%) and 2 (5%) patients, respectively. Baseline alfa-fetoprotein was elevated (>6 ng/mL) in 3 (7%) patients. DNAJB1-PRKACA fusion mutation was evaluated in two patients and was present in both. Other gene mutations identified included ATR V56fs*11 and LZTR1 F720fs*47(1 patient), mosaic heterozygous deletions of 1p, chromosomes 4, 14, 15q, 18, 21, 22, X (1 patient) and EGFR mutation (1 patient). One patient had programmed cell death ligand-1 (PD-L1) expressing tumor with 10% of tumor cells being positive for PD-L1.
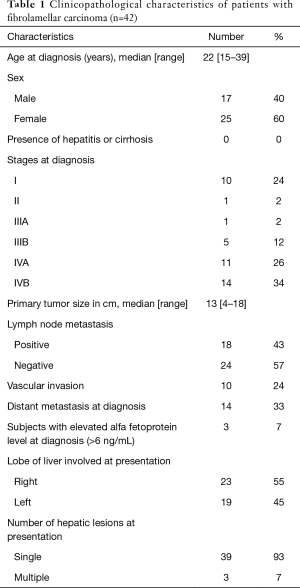
Full table
Treatment
A total of 31 out of 42 patients (74%) underwent surgical resection at presentation. Among the patients who underwent surgical resection, 10 patients had stage I disease with median tumor size of 11 cm (range, 3.5 to 14 cm); one patient had stage II disease, four were of stage III disease. Sixteen of 25 patients (64%) of stage IV disease had surgical resection of the primary tumor. Nine of the 10 patients (90%) with stage I disease had negative (R0) surgical margins where as 75% (3 of 4 patients) of stage III and 43% (7 out of 16 patients) of stage IV had negative (R0) surgical resection. Systemic therapy was given to 9 of these 31 resectable patients: as neo-adjuvant therapy in 1 patient (5-flurouracil + interferon for 4 months) and post-surgical therapy in 8 patients. Post-surgical therapy consisted of cisplatin + doxorubicin + 5-flurouracil in 1 patient, cisplatin + doxorubicin in 1 patient, gemcitabine and oxaliplatin in 1 patient, 5-flurouracil + interferon in 1 patient and sorafenib in 4 patients.
Systemic therapy was administered in 17 patients including those with unresectable disease at presentation or who developed recurrences post-surgery. Systemic treatment regimens included sorafenib, FOLFOX (5-fluorouracil/leucovorin and oxaliplatin), gemcitabine plus oxaliplatin, doxorubicin, gemcitabine, capecitabine plus interferon alfa, gemcitabine plus cisplatin, cisplatin plus doxorubicin and nivolumab. Sorafenib was administered in 9 patients and 4 patients achieved stable disease (SD) with duration ranging from 5 months to 5 years (median 8 months). Another patient achieved a SD with doxorubicin lasting for 9 months. One patient with PD-L1 expressing tumor had a complete response (CR) after 2 months of therapy with nivolumab and remains in CR after 11 months. The responses to various forms of systemic therapy are summarized in Table 2.
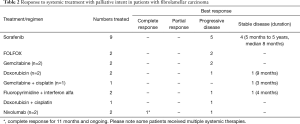
Full table
Survival outcome
Median OS of the entire cohort was 38 months. For stage I patients (n=10), Kaplan-Meier survival analysis revealed a 5-year OS of 86%. There were six recurrences out of 10 patients in stage I group with a median time to recurrence of 30.5 months (range, 10–120 months). Median OS of the patients with unresectable tumors (n=11) was 10 months (range, 3–52 months). Of the 31 patients who underwent surgery upfront, five patients had residual disease. Of all patients who underwent surgery at diagnosis (n=31), 22 patients developed recurrent disease, indicating an overall recurrence rate of 71%. The patients who had undergone any form of resection had a better median OS as compared to the patients who were unresectable (73 vs. 10 months; P<0.0001) (Figure 1). On sub-group analysis, for stage IV patients who underwent resection at presentation (n=16), 5-year OS was 35% and a median OS of 38 months (range, 8–336 months), which was significantly inferior compared to stage I-III patients (median OS: not reached; 5-year OS: 74%) (P=0.02) (Figure 2). No significant OS difference was noted between the patients with or without nodal metastasis at the time of presentation (29 vs. 51 months, respectively; P=0.27). It is to be noted that the difference could be clinically meaningful, and perhaps may not have reached statistical significance due to small sample size in subgroups. Similarly, we did not notice statistically significant difference in OS between males and females (34 vs. 38 months; P=0.51).
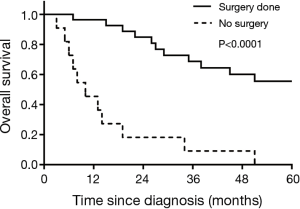
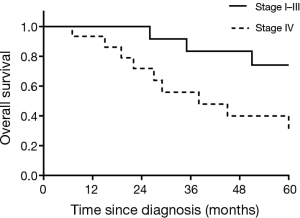
Of the 31 patients who underwent resection at presentation, 9 patients received peri-operative systemic therapy. However, peri-operative systemic therapy did not significantly change OS when adjusted for stage (HR of 1.39, 95% CI: 0.43–4.41, P=0.57).
Discussion
Although first described in 1956 by Edmondson, given the rarity of the disease, minimal progress has been made in identifying effective therapeutic regimens for the management of FLHCC (15). FLHCC represents less than 1% of all primary liver tumors diagnosed in the United States (16). FLHCC typically affects younger individuals, 5 to 35 years of age, although there appears to be two peak incidences: one at age 10 to 30 years and another at age 70 to 79 years (17). In a large database series, the average age at diagnosis was 39 years, and 60 percent of patients were diagnosed under the age of 40 (12). In series reported from large academic centers and in ours, median age of diagnosis varied from 20 to 32 years (6,7) and did not include any data on patients older than 70 years, possibly reflecting a referral bias as all these reports originated from tertiary care centers specializing in liver surgery.
Most of the clinicopathological data and survival outcome are derived from case reports, single-institutional case series and registry-based studies (6-8,10-14,18). Table 3 summarizes recently published series including the current case series. Although previous single institutional studies have showed male preponderance, we have seen a slight female predilection in our cohort (60% females) which was also reported in a National Cancer Data Base analysis and a multi-institutional study (6,19). In this study, we noted that female patients had a clinically meaningful better median OS (73 months) as compared to that of males (34 months) but did not reach statistical significance, which may be attributed to small sample size. Better survival in female patients was reported in another single institutional study (85 vs. 42 months, P=0.10) (20) and a SEER registry-based analysis (21), whereas a multi-institutional study showed opposite result (6). These discordant results may be stemming from the heterogeneous nature of the disease and small sample size in the studies, which again may be attributed to rarity of the disease.

Full table
Consistent with previous studies, approximately 60% of the patients had stage IV disease at the time of diagnosis (6,22). In addition, we found that lymph nodes were the most common sites of metastasis (6,22). Lymph node metastasis has been shown to be associated with worse outcomes in prior studies (20), although our series did not show statistically significant difference (P=0.27).
Surgical therapy has a proven benefit in improving OS as noted in the present and previously published reports. However, recurrence rate is quite high in spite of R0 resection. In our cohort, we observed a 71% recurrence rate in the patients who had surgical resection (stage I to stage IV) at presentation. It is to be noted that in our study 74% of the patients who underwent surgical resection had negative (R0) surgical margins. Moreover, of the 10 stage I patients, 6 patients had recurrence. Similar high recurrence rate has been reported in other large series as well. These findings, considered together, suggest that surgery is unlikely to cure majority of FLHCC patients, even in early stage disease, which underscores the need and importance of systemic therapy in the management of FLHCC.
No adjuvant or neoadjuvant systemic therapies have been reported to demonstrate survival benefit in resectable FLHCC patients. Analysis of our series showed peri-operative systemic therapy did not significantly change OS. In addition, information on systemic therapy in the patients who are not candidates for resection is sparse and no systemic therapy has shown consistent benefit. In our study, we noticed that sorafenib resulted in stable disease in 4 out of 9 patients with benefit lasting from 5 months to 5 years (Table 2). In another report, sorafenib was administered in 10 patients; among which eight had disease progression after 2.5 to 7 months of treatment, one had a mixed response followed by progression, and one was lost to follow-up (6). We noticed a durable complete response lasting for 11 months and ongoing with nivolumab in a patient who had a PD-L1 expressing tumor. Chemotherapy has demonstrated limited activity in this disease with 5-fluorouracil based therapy being most commonly used regimen. Kaseb et al. reported an improvement in median OS with 5-fluorouracil and interferon (20). In their retrospective analysis, the combination of 5-flurouracil and interferon resulted in stable disease in 37% (10 out of 27) of patients while a complete response was noted in 7% (2 out of 27). In our case series, 2 patients received the combination of 5-flurouracil and interferon and 1 patient achieved stable disease for 4 months.
Study limitations are inherent to retrospective study design and relatively small sample size of the cohort. Nonetheless, the notable feature of the present study is availability of systemic therapy details and having the details on the patient response to the treatment (Table 2). The role of systemic therapy in FLHCC has not been evaluated extensively, and the current literature shows conflicting results with different modalities of therapy given the rarity of the cancer (20,23-25).
Conclusions
Patient demographic features and survival patterns demonstrated in the analysis are consistent with that of previously published reports. However, the current study added more insight into the therapeutic outcomes of various treatment modalities in FLHCC. We found that FLHCC has high recurrence rate irrespective of the stage of disease at diagnosis and resectability. PD-L1 expression in FLHCC might render support to consideration of potential utilization of checkpoint inhibitors. Future studies that evaluate various therapeutic strategies targeting molecular pathways and genetic alterations would help better understanding of the natural history of this disease thereby improving the OS.
Acknowledgements
None.
Footnote
Conflicts of Interest: Part of the work is accepted for poster presentation in annual proceedings of American Society of Clinical Oncology (ASCO)-GI symposium January 2019.
Ethical Statement: The study protocol has been approved by the Mayo Clinic Committee on human research (IRB number: 17-008561) and written informed consent was obtained from all patients.
References
- Kassahun WT. Contemporary management of fibrolamellar hepatocellular carcinoma: diagnosis, treatment, outcome, prognostic factors, and recent developments. World J Surg Oncol 2016;14:151. [Crossref] [PubMed]
- Van Eyken P, Sciot R, Brock P, et al. Abundant expression of cytokeratin 7 in fibrolamellar carcinoma of the liver. Histopathology 1990;17:101-7. [Crossref] [PubMed]
- Ward SC, Huang J, Tickoo SK, et al. Fibrolamellar carcinoma of the liver exhibits immunohistochemical evidence of both hepatocyte and bile duct differentiation. Mod Pathol 2010;23:1180-90. [Crossref] [PubMed]
- Ross HM, Daniel HD, Vivekanandan P, et al. Fibrolamellar carcinomas are positive for CD68. Mod Pathol 2011;24:390-5. [Crossref] [PubMed]
- Graham RP, Yeh MM, Lam-Himlin D, et al. Molecular testing for the clinical diagnosis of fibrolamellar carcinoma. Modern Pathology 2018;31:141. [Crossref] [PubMed]
- Ang CS, Kelley RK, Choti MA, et al. Clinicopathologic characteristics and survival outcomes of patients with fibrolamellar carcinoma: data from the fibrolamellar carcinoma consortium. Gastrointest Cancer Res 2013;6:3-9. [PubMed]
- Chagas AL, Kikuchi L, Herman P, et al. Clinical and pathological evaluation of fibrolamellar hepatocellular carcinoma: a single center study of 21 cases. Clinics (Sao Paulo) 2015;70:207-13. [Crossref] [PubMed]
- Groeschl RT, Miura JT, Wong RK, et al. Multi-institutional analysis of recurrence and survival after hepatectomy for fibrolamellar carcinoma. J Surg Oncol 2014;110:412-5. [Crossref] [PubMed]
- Honeyman JN, Simon EP, Robine N, et al. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science 2014;343:1010-4. [Crossref] [PubMed]
- Wahab MA, El Hanafy E, El Nakeeb A, et al. Clinicopathological features and surgical outcome of patients with fibrolamellar hepatocellular carcinoma (experience with 22 patients over a 15-year period). World J Gastrointest Surg 2017;9:61-7. [Crossref] [PubMed]
- Yamashita S, Vauthey JN, Kaseb AO, et al. Prognosis of Fibrolamellar Carcinoma Compared to Non-cirrhotic Conventional Hepatocellular Carcinoma. Journal of Gastrointestinal Surgery 2016;20:1725-31. [Crossref] [PubMed]
- Eggert T, McGlynn KA, Duffy A, et al. Fibrolamellar hepatocellular carcinoma in the USA, 2000-2010: A detailed report on frequency, treatment and outcome based on the Surveillance, Epidemiology, and End Results database. United European Gastroenterol J 2013;1:351-7. [Crossref] [PubMed]
- Kakar S, Burgart LJ, Batts KP, et al. Clinicopathologic features and survival in fibrolamellar carcinoma: comparison with conventional hepatocellular carcinoma with and without cirrhosis. Mod Pathol 2005;18:1417-23. [Crossref] [PubMed]
- Nagorney DM, Adson MA, Weiland LH, et al. Fibrolamellar hepatoma. Am J Surg 1985;149:113-9. [Crossref] [PubMed]
- Edmondson HA. Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. AMA J Dis Child 1956;91:168-86. [PubMed]
- El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatology Research 2007;37:S88-94. [Crossref] [PubMed]
- Eggert T, McGlynn KA, Duffy A, et al. Epidemiology of fibrolamellar hepatocellular carcinoma in the USA, 2000-10. Gut 2013;62:1667-8. [Crossref] [PubMed]
- Kaseb AO, Shama M, Sahin IH, et al. Prognostic indicators and treatment outcome in 94 cases of fibrolamellar hepatocellular carcinoma. Oncology 2013;85:197-203. [Crossref] [PubMed]
- Jernigan PL, Wima K, Hanseman DJ, et al. Natural history and treatment trends in hepatocellular carcinoma subtypes: Insights from a national cancer registry. J Surg Oncol 2015;112:872-6. [Crossref] [PubMed]
- Kaseb AO, Shama M, Sahin IH, et al. Treatment outcome and prognostic indicators in 94 cases of fibrolamellar hepatocellular carcinoma. Oncology 2013;85:197-203. [Crossref] [PubMed]
- Mayo SC, Mavros MN, Nathan H, et al. Treatment and Prognosis of Patients with Fibrolamellar Hepatocellular Carcinoma: A National Perspective. J Am Coll Surg 2014;218:196-205. [Crossref] [PubMed]
- Stipa F, Yoon SS, Liau KH, et al. Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer 2006;106:1331-8. [Crossref] [PubMed]
- Malouf GG, Brugières L, Le Deley MC, et al. Pure and mixed fibrolamellar hepatocellular carcinomas differ in natural history and prognosis after complete surgical resection. Cancer 2012;118:4981-90. [Crossref] [PubMed]
- Katzenstein HM, Krailo MD, Malogolowkin MH, et al. Fibrolamellar hepatocellular carcinoma in children and adolescents. Cancer 2003;97:2006-12. [Crossref] [PubMed]
- El-Gazzaz G, Wong W, El-Hadary MK, et al. Outcome of liver resection and transplantation for fibrolamellar hepatocellular carcinoma. Transpl Int 2000;13 Suppl 1:S406-9. [Crossref] [PubMed]


