Relationship of ethnicity and overall survival in patients treated with sorafenib for advanced hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is one of the most frequent cancers worldwide with approximately 600,000 deaths annually (1) and it usually occurs in the setting of chronic liver disease and cirrhosis. While local therapies play a major role in the treatment of it HCC often progresses to advanced stages where the primary treatment is systemic therapy. The only approved systemic therapy for patients with advanced HCC is sorafenib, an oral multikinase inhibitor that blocks tumor cell proliferation by targeting Raf/MEK/ERK signaling at the level of Raf kinase. It also exerts antiangiogenic effect by targeting vascular endothelial growth factor receptor-2/-3 [vascular endothelial growth factor receptor-2/-3 (VEGFR-2/-3)], and platelet derived growth factor receptor beta (PDGFR) tyrosine kinases (2).
Given the encouraging results with sorafenib in phase I and II trials, the multicenter, phase III, double-blind, randomized, placebo-controlled SHARP trial was conducted among 602 patients with advanced HCC in the Western population (3). The study was stopped early at the second planned interim analysis due to an overall survival (OS) advantage favoring sorafenib [median OS of 10.7 months vs. 7.9 months, with a hazard ratio of 0.69; 95% confidence interval (CI), 0.55 to 0.87; P<0.001]. Because the SHARP study did not include the Asia-Pacific region, where chronic hepatitis B viral (HBV) infection is an important etiological factor, another phase III trial comparing sorafenib and placebo was conducted among Asian patients with advanced HCC (4). Despite showing an improved median OS in the sorafenib arm of 6.5 months compared with 4.2 months in those who received placebo, the median OS observed was inferior to that in the SHARP trial, even though both studies used similar inclusion and exclusion criteria.
One potential explanation is that the Asia-Pacific trial results were less favourable due to the fact that the patients were more ill at baseline than those in the western study, with a generally worse performance status and more advanced stage of disease. For instance, 53.9% of the patients in the SHARP study had Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 as compared to 26.1% in the Asian trial (3,4). Chronic HBV, acquired through vertical transmission during childbirth or horizontal transmission in early childhood, is the most common cause of liver cancer among Asians, except in Japan where hepatitis C viral (HCV) infection is the most common etiology (5,6). Therefore, Asian patients have a higher prevalence of HBV-related HCC as compared to Western populations. Seventy-three percent of the patients enrolled in the Asian trial had HBV infection versus only 18% of those enrolled to SHARP study. Moreover, some exploratory analyses suggest that patients with HCV infection as the etiology of their cirrhosis may have a better response to sorafenib as compared to those with other underlying causes of cirrhosis (7,8).
It remains unclear whether this disparity in outcomes between Caucasian and Asian patients is a result of variations in liver disease etiology, pharmacogenetic differences between Western and Asian populations, diverse loco-regional therapy for early-stage disease, or a combination of these many factors. Given the fact that the province of British Columbia (BC) has a sizeable Asian population, it offers an opportunity for comparison to non-Asian patients with HCC. Moreover, patients are treated at the same institution, providing a rationale for investigating whether ethnicity or etiology of hepatitis are associated with poor outcomes. The aim of this study was to investigate whether ethnicity conferred differences among patients who received sorafenib for advanced HCC.
Methods
A total of 255 consecutive patients with advanced HCC who initiated sorafenib from January 2008 to February 2013 were identified using the BC pharmacy database. BC is a Canadian province with a population of approximately 4.4 million, and two thirds of all systemic cancer treatments are delivered in five major cancer centers and their satellite clinics. Eligibility for the funded use of sorafenib in BC for advanced HCC is typically restricted to patients with Child-Pugh A disease and ECOG PS of 0-2.
Clinicopathological variables and outcomes were retrospectively collected. Ethnicity was categorized into Asian versus non-Asian. Ethnicity was defined primarily based on information contained in the patient’s medical record, taking into consideration self-identification by the patient, or inference using race/ethnicity of parents, birthplace, or family name. All patients diagnosed with HCC are tested for HBV and HCV in our institution. Patients were considered HBV or HCV positive if there was serology confirmation of infection.
Statistical analysis
Statistical analysis was performed using SPSS version 14.0 for Windows® (SPSS, Chicago, IL, USA). Baseline characteristics were compared between Asians and non-Asians by using Chi-test for categorical variables and F test for continuous variables. OS was calculated in months from the time of primary diagnosis to date of death or censored at last follow-up. Progression free survival (PFS) was calculated from the time of primary diagnosis to date of disease progression according to the medical notes from the attending physician as well as imaging reports from local radiologists. Kaplan-Meier curves for OS were generated. The log-rank test was used to assess statistical differences among variables and P values <0.05 were considered statistically significant.
Multivariable survival analyses were performed using Cox proportional hazards regression models to determine the association between ethnicity and OS outcomes after adjustment for potential confounders. Variables in the model included ethnicity, status of HBV, status of HCV, ECOG PS and presence of cirrhosis. Hazard ratios (HR) and 95% CI were calculated to estimate risk of death.
Results
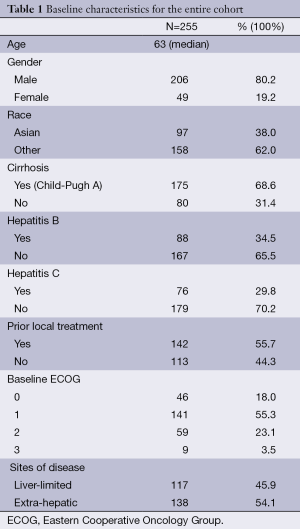
Two hundred and fifty five patients were included in the analysis. Median follow-up time was 23.6 months. Baseline characteristics of the entire cohort are described in Table 1. Median age was 63 years (range, 22-93 years) and median AFP prior to starting on sorafenib was 248 ng/mL (range, 1.3-720,000 ng/mL). The majority consisted of male (80.2%). Ninety-seven patients (38%) had Asian background and 158 (62%) were non-Asians.
Full table
Baseline characteristics were comparable among Asians and non-Asians, except for HCV, HBV and presence of cirrhosis (Table 2). There was a higher proportion of HCV positivity and cirrhosis among non-Asians while Asians accounted for the majority of HBV positivity. Among all patients, 34.5% had HBV and 29.8% had HCV. Five patients (2%) were positive for both HBV and HCV. In addition, 68.4% had cirrhosis (all of them Child-Pugh A) and 46.3% had liver-limited disease.
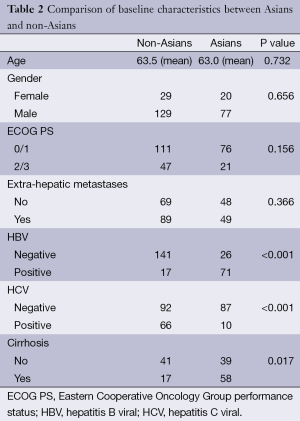
Full table
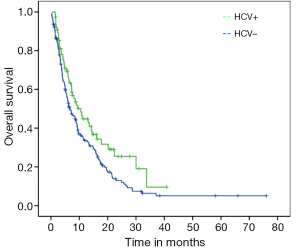
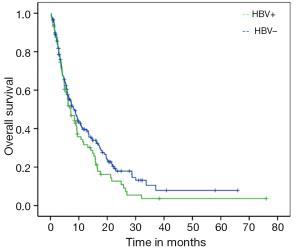
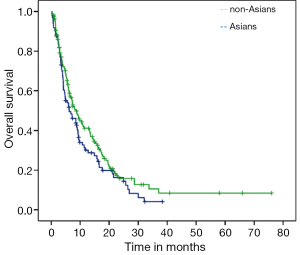
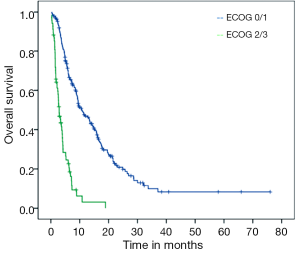
Median PFS for the entire cohort was 3.7 months (95% CI: 3.33-4.20) while median OS from initiation of sorafenib to death was 7.5 months (95% CI: 5.72-9.27). On univariate analysis, good ECOG PS and history of HCV were significantly associated with better OS. Median OS for HCV positive patients was 10.5 months as compared to 6.6 months for those with no history of HCV (P=0.03; HR=0.69) (Figure 1). Median OS for patients with either ECOG 0 or 1 was 10.7 months while patients with ECOG PS≥2 achieved only 2.8 months (P<0.001; HR=0.21) (Figure 2). Age, gender, HBV positivity (Figure 3), ethnicity (Figure 4), presence of cirrhosis and extra-hepatic metastases were not significantly associated with OS. Median OS for HBV positive patients was 7.1 months as compared to 8.1 months for those with no history of HBV (P=0.123) (Figure 3). Asian patients had a median OS of 6.2 months while non-Asians achieved 8.5 months (P=0.131) (Figure 4).
On multivariate analysis, only good ECOG PS and HCV positivity correlated with better OS (P<0.001; HR=0.19 and P=0.04; HR=0.66, respectively) (Table 3). Asian ethnicity had no independent influence on OS (HR=1.10, 95% CI: 0.74-1.63; P=0.62).
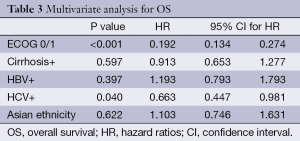
Full table
Discussion
The results of our study do not support the hypothesis that Asian ethnicity is independently associated with inferior survival on sorafenib as compared to non-Asian patients. We have demonstrated that HCV positivity and good ECOG PS are associated with better OS, while ethnicity and HBV are not. Given the fact that non-Asian patients are more likely to be HCV positive, this may be the confounding variable most responsible for the associated inferior survival among Asian patients treated with sorafenib. While the SHARP trial included 28% of patients with HCV and 18.4% with HBV, the Asian trial had a much higher proportion of HBV (73%) and only 8.4% of patients with HCV (3,4). Moreover, an unplanned retrospective analysis of SHARP study showed that the subset of patients with HCV-related HCC had a more favorable median OS than the general population in the study (14 vs. 10.9 months) (7). In the present study, the median OS for HCV-positive group was also significantly prolonged when compared to HCV-negative patients (10.5 vs. 6.6 months; P=0.03; HR=0.69).
A prior small retrospective analysis was conducted among 46 patients (13 HCV+, 33 HBV+) with HCC who received sorafenib as part of a phase II trial. The authors looked for a difference between HBV (n=33) and HCV (n=13) related HCC (9). Although no statistical significant differences in OS and median PFS were found, there was a tendency towards a better time to progression in HCV patients vs. HBV patients (6.5 vs. 4 months, respectively), suggesting that sorafenib may be more efficacious in HCV-related HCC (9). HCV core proteins are known to induce a high basal rate of Raf-1 activity, an important kinase involved in the oncogenesis of HCC. It is hypothesized that there may be significant differential expression of Raf-1 among HCV and HBV-related HCC (9,10). Due to the fact that sorafenib effectively inhibits Raf-1 kinase, it may thereby slow down the progression of HCV-related HCC more efficaciously, as compared to that of HBV.
A recent systematic review of prospective studies has explored the efficacy and safety of sorafenib for treating advanced HCC (11). According to the authors, HBV infection might adversely influence the response to sorafenib. The included studies with the highest percentages of patients with HBV showed the lowest OS and disease control rates (DCR). For instance, a phase II trial from Hong Kong had the highest percentage of HBV patients (90%) and described the lowest OS (5 months) and DCR (26%) (12). In contrast, a Japanese phase I trial, in which 74% of patients had HCV, had the longest OS (15.6 months) and the highest DCR (82%) (13). In addition to this systematic review, a subgroup analysis of the SHARP trial confirmed that sorafenib consistently improved median time to tumor progression in all subgroups, except in HBV-positive patients. However, those results were limited by small patient numbers in some subsets, such as the HBV-positive group (8).
The Global Investigation of Therapeutic Decisions in HCC and of its Treatment with Sorafenib (GIDEON) study is a large prospective, non-interventional study undertaken in more than 3,000 patients with unresectable HCC from 39 countries (14). Differences in the etiology of underlying liver disease were observed across regions. For instance, the majority of patients in Asia-Pacific had HBV infection (84%), whereas HCV infection was more common in Europe (33%) and the USA (50%). A greater proportion of patients in Europe (42%) and the USA (34%) had alcoholic liver disease compared with other regions (16-19%). However, outcomes according to ethnicity or hepatitis infection are still not available.
It is believed that ethnic diversity in drug response are determined by environmental factors (that influence bioavailability and metabolism, such as the frequency of smoking, alcohol drinking, herbal medicine use and local dietary varieties), local medical care preferences, variability of genetic polymorphisms in drug metabolizing enzymes and transporters, and the prevalence of an ethnically restricted mutations in a drug’s receptor/target that may cause a particular sensitivity or resistance to that drug (15). To date, there is no evidence that response to sorafenib is related to ethnic diversity and our study supports this notion.
Our study has some notable strength. The availability of a sizeable Asian migrant population in the province of BC made this study possible. The fact that both Asian and non-Asian patients are treated at the same institution with access to sorafenib approved in accordance with defined criteria for use, increases the strength of this analysis due to standard patterns of oncology practice. Nonetheless, our results should be interpreted in the context of several limitations. First, the retrospective, non-randomized nature of this review relies on accuracy of written records and information captured by them. Second, we were unable to ascertain the exact cause of death of our cohort of patients since death certificates are not captured systematically. It was also not possible to determine the exact cause of underlying liver disease other than HBV or HCV. Moreover, we were unable to evaluate toxicity and discontinuation rates, which would otherwise strengthen our results.
Conclusions
According to our findings, differences in outcomes for patients with advanced HCC treated with sorafenib are associated with the etiology of the underlying liver disease rather than ethnicity. When treated with sorafenib at the same institution, Asians and non-Asians with advanced HCC had similar OS. Good ECOG PS and HCV positivity were the only significant favourable prognostic factors. A higher proportion of HCV positivity may explain why the SHARP trial achieved better OS when compared to the Asian-Pacific trial. Further randomized studies are needed to establish the influence of underlying liver disease on sorafenib treatment response.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [PubMed]
- Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099-109. [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [PubMed]
- Nguyen TT, Taylor V, Chen MS Jr, et al. Hepatitis B awareness, knowledge, and screening among Asian Americans. J Cancer Educ 2007;22:266-72. [PubMed]
- Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: consider the population. J Clin Gastroenterol 2013;47 Suppl:S2-6. [PubMed]
- Bolondi L, Caspary W, Bennouna J, et al. Clinical benefit of sorafenib in hepatitis C patients with hepatocellular carcinoma (HCC): subgroup analysis of the SHARP trial (abstract). Data presented at the 2008 ASCO Gastrointestinal Cancers Symposium. Orlando, Florida, USA, 2008:abstr 129.
- Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 2012;57:821-9. [PubMed]
- Huitzil-Melendez FD, Saltz LS, Song J, et al. Retrospective analysis of outcome in hepatocellular carcinoma (HCC) patients (pts) with hepatitis C (C+) versus B (B+) treated with sorafenib (S). 2007 ASCO Gastrointestinal Cancers Symposium. Orlando, Florida, USA, 2007.
- Giambartolomei S, Covone F, Levrero M, et al. Sustained activation of the Raf/MEK/Erk pathway in response to EGF in stable cell lines expressing the Hepatitis C Virus (HCV) core protein. Oncogene 2001;20:2606-10. [PubMed]
- Xie B, Wang DH, Spechler SJ. Sorafenib for treatment of hepatocellular carcinoma: a systematic review. Dig Dis Sci 2012;57:1122-9. [PubMed]
- Yau T, Chan P, Ng KK, et al. Phase 2 open-label study of single-agent sorafenib in treating advanced hepatocellular carcinoma in a hepatitis B-endemic Asian population: presence of lung metastasis predicts poor response. Cancer 2009;115:428-36. [PubMed]
- Furuse J, Ishii H, Nakachi K, et al. Phase I study of sorafenib in Japanese patients with hepatocellular carcinoma. Cancer Sci 2008;99:159-65. [PubMed]
- Lencioni R, Kudo M, Ye SL, et al. First interim analysis of the GIDEON (Global Investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafeNib) non-interventional study. Int J Clin Pract 2012;66:675-83. [PubMed]
- O’Donnell PH, Dolan ME. Cancer pharmacoethnicity: ethnic differences in susceptibility to the effects of chemotherapy. Clin Cancer Res 2009;15:4806-14. [PubMed]