
Status of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis from colorectal cancer
Introduction
Peritoneal carcinomatosis (PC) is a major concern in the management of advanced intra-abdominal cancer. PC causes severe suffering and is associated with abdominal distension, pain, malnutrition, ascites, cachexia, and intestinal obstruction. PC from colorectal cancer (CRC) occurs in 20% of patients with non-mucinous CRC, presenting a synchronous or metachronous appearance, of which 6–8% is peritoneal only. In contrast, about 50% of patients with mucinous and signet ring cell tumors develop PC (1). PC is a lethal condition in cancer, with high morbidity and mortality rates, and is the most common cause of death after primary intra-abdominal tumor resection (2-4). The traditional treatment strategy for PC from CRC was systemic chemotherapy with or without surgery to alleviate symptoms (5). However, the distinctive characteristics of peritoneum, including a poor blood supply to the peritoneal surface with low penetration into tumor nodules, led to a poor chemotherapeutic efficacy for PC compared to that for solid organ metastasis (6,7).
Over the last century, management of PC from CRC has evolved to prolong survival and improve patient quality of life with the development of new chemotherapeutic agents, surgical techniques, and further understanding of biology of PC. Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) was independently developed as a treatment modality for PC of ovarian origin and eventually to other various gastrointestinal cancers. HIPEC in combination with CRS has since played as a crucial role and changed the paradigm in the management of PC. Despite concerns about high morbidity and mortality after CRS with HIPEC, it may provide long-term survival by controlling tumor burden and improving symptoms in select patients (8-11). While thousands of articles report on CRS with HIPEC in various institutions worldwide, the limitations include a lack of definitive well-designed clinical trials and variation in techniques across institutions.
This review details the most recent knowledge, ongoing investigations, and potential directions for the treatment of PC from CRC.
History of CRS with HIPEC
CRS and HIPEC were independently developed as treatment modalities for peritoneal metastasis of ovarian origin and eventually for other gastrointestinal cancers. In the 1930s, debulking surgery was initially described for locally advanced ovarian cancers (12). After recognizing that peritoneum is a hypoxic environment with poor penetration by systemic chemotherapy, intraperitoneal chemotherapy was applied to PC of not only ovarian cancer but also other gastrointestinal cancers (13,14). In the 1980s, hyperthermia was increasingly used in intraperitoneal chemotherapy to increase the efficacy and potency of the anti-neoplastic agents (15). HIPEC in conjunction with CRS has since emerged as an essential means of controlling peritoneal disease and has been associated with favorable survival outcomes in selected patients.
Development of CRS
CRS was first proposed in 1934 by Dr. Meigs with the notion that tumor debulking surgery for ovarian cancer under the premise that reducing macroscopic disease burden would improve patient symptoms and reduce complications (12). In the late 1960s and 1970s, Munnell and Griffiths reported that extensive tumor debulking surgery in ovarian cancer improved survival rates and that residual tumor mass size <1.6 cm after CRS was significantly associated with better survival (16,17). In 1969, Long et al. reported superior survival rates in five of 17 PC patients with pseudomyxoma peritonei who underwent multiple cytoreductive procedures combined with the use of alkylating agents (intraperitoneal or oral) (18). Similar results were reported following extensive experiences by the Memorial Sloane-Kettering Cancer Center in 1950–1970 (19). They suggested that aggressive CRS offered the best chance for patient survival, whereas radiation and systemic chemotherapy did not affect prognosis. Aggressive CRS, therefore, was adopted as the mainstay for management and palliation.
Development of intraperitoneal chemotherapy with hyperthermia
In 1970s, due to increased insight into the pharmacokinetic differences between IV and intraperitoneal tumors, impact of systemic and total body hyperthermia on patients with advanced cancers was being investigated for its effects on decreasing overall tumor burden based on the early positive results of isolated perfusion of visceral vasculature with hyperthermic chemotherapy (41 to 42 °C) from 1960s (20,21). Restriction of perfusion to specific body areas for cancer chemotherapy was based on the belief that this would be more effective than systemic administration since neoplastic tissue would be exposed to a higher drug concentration (22). Palta developed a thermal infusion filtration system for the intraperitoneal infusion of chemotherapeutic agents (23), to manage malignant effusions and treat metastatic cancers of the intracavitary serosa, which was deemed safe for clinical procedures. Hyperthermia induced nonlethal responses in physiology. In 1979, Spratt used the thermal transfusion infiltration system (TIFS) to successfully deliver hyperthermic chemotherapy into the peritoneal space of a patient with locally advanced abdominal malignancy (13,14). The patient underwent CRS followed by TIFS to deliver heated intraperitoneal thiotepa, and was then administered intraperitoneal methotrexate on postoperative day 5 through catheters. The patient survived the operation and was discharged without significant complications. The development of CRS with postoperative intraperitoneal chemotherapy continued in the early 1980s when Sugarbaker investigated its therapeutic efficacy in patients with peritoneal metastases of not only ovarian cancer but also various gastrointestinal tumors (15). He emphasized that locoregional cancer treatments may be more beneficial to patients than systemic therapies if the following three criteria were met. (I) Systemic benefits of treatment were not sacrificed because adequate doses of the drug are administered locally so that therapeutic amounts are present within the peripheral circulation. (II) Higher levels of effective chemotherapeutic agents are present local-regionally so that local anticancer effects are increased. (III) Local and systemic toxic side effects are no greater than those when the drugs are administered intravenously. Several effective anticancer agents meeting these criteria are used intraperitoneally for the treatment of gastrointestinal malignancy.
In 1987, Japanese surgeons reported positive results of intraperitoneal hyperthermic perfusion with mitomycin C and a thermosensitizing drug (misonidazole) during debulking surgery in a group of 15 patients with advanced gastric cancer (24). In 1995, Sugarbaker definitively systematized the rationale and surgical technique for peritonectomy (25). Since then, CRS and HIPEC have been further developed and performed by several medical centers worldwide in patients with various peritoneal-surface-malignancies, including PC, sarcomatosis, and peritoneal mesothelioma.
Patient selection criteria
Patient selection is one of the most challenging issues in CRS with HIPEC in PC from CRC. The common indication for PC from CRC to be treated with CRS with HIPEC is limited to the abdomen which is completely or significantly resectable. General contraindications for CRS and HIPEC are (I) age >70 years; (II) serious medical histories; (III) clinical aggravation with systemic chemotherapy; (IV) malnutrition; (V) concomitant extra-abdominal metastasis; (VI) unresectable liver metastases (LM); and (VII) massive retroperitoneal bulk disease or lymph node involvement (2,26). However, there are no clear definitions of patient selection criteria. Qualitative and quantitative indicators are mandatory to evaluate patient eligibility.
Qualitative indicators
Several clinical and histopathological risk factors have been identified as risk factors for synchronous PC from CRC. These include an advanced T stage, lymph node metastases, and a poor differentiation grade (27).
Since the prevention of metachronous PC is more encouraging than that for synchronous PC, there are currently more studies on the risk factors for metachronous metastases. According to several studies, the independent risk factors for metachronous PC include advanced T and N stages, malignant ascites, perforate cancer, tumor differentiation, solid organ metastasis, and peritoneal fluid cytology (2,28,29).
Patients with synchronous PC and LM treated with palliative intent had poor survival outcomes (30). However, over the last decade, a number of studies have reported on patients treated with CRS and HIPEC combined with local treatment of LM (31-35). Current literature and available meta-analysis data suggest that combining CRS and HIPEC with curative treatment for LM results in improved survival compared to that with treatment with modern systemic chemotherapy alone, although survival was worse than that in a peritoneal metastasis-only group treated CRS and HIPEC (31). However, patient selection according to the extent of metastasis is essential. Several studies have assessed the effectiveness CRS with HIPEC in patients with PC with or without LM (32-35). In those studies, all liver procedures, including major hepatectomy, were performed according to liver mass. A case-control study of 37 patients with PC and LM matched with 61 patients with PC alone showed that prolonged survival could be achieved in highly selected patients operated on for limited PC and fewer than three LM (35). Elias et al. also reported that the combined treatment of LM plus PC was beneficial in selected patients presenting three or fewer metastases (33). In a study on the safety of synchronous liver resection and CRS with HIPEC, patients who underwent CRS with HIPEC plus liver resection underwent a higher number of operative procedures, had a longer operative time, had a longer hospital length of stay, were more likely to require reoperation, and experienced greater 30-day morbidity but not mortality. Therefore, safety and oncologic outcomes must also be considered (36).
The prognosis of patients with PC from CRC is strongly influenced by histological types regardless of treatment (37). The adverse prognostic impact of histological subtype has been reported in patients treated with CRS and HIPEC (38,39). In several retrospective studies, the median survival times did not exceed 13 months after CRS with HIPEC. Additionally, several studies identified signet ring cell histology as a significant prognostic factor, with hazard ratios (HR) ranging from 2.0 to 3.7 (40-42).
Stratifying high-risk patients with metachronous regional disease with gross and histologic characteristics would be of benefit as these patients may benefit from preventative techniques such as a prophylactic HIPEC and surveillance with second-look surgery, which are currently under investigation.
Roles of radiologic evaluation and diagnostic laparoscopy
Radiologic evaluation before CRS with HIPEC may allow the determination of the extent not only of intra-abdominal but also extra-abdominal disease. The Fifth International Workshop on Peritoneal Surface Malignancy in Milan reported the consensus on preoperative investigations for peritoneal surface malignancy (28). They concluded that contrast-enhanced multi-sliced computed tomography (CT) was the fundamental imaging modality, whereas magnetic resonance imaging (MRI), positron emission tomography (PET), laparoscopy, and serum tumor markers (STMs) were considered useful but not fundamental investigational modalities.
CT is the preferred tool for selecting patients for surgery because of the ease to perform, shorter imaging times, fewer movement artifacts caused by bowel peristalsis, higher spatial resolution, accessibility and familiarity of radiologists and clinicians. However, standard surveillance with CT has a low sensitivity for small peritoneal nodules, at an estimated less than 30% for nodules <0.5 cm in size (29). Numerous studies have shown a variable correlation between CT prediction and intraoperative findings, indicating that CT tends to underestimate the clinical peritoneal carcinomatosis index (PCI) scores (43). Thus, more studies support the role of 2-fluorodeoxyglucose (FDG)/PET for the detection of PC (44). The advantage of PET/CT is whole body coverage, providing information about the presence of distant metastases that exclude the patient from CRS with HIPEC (45). However, in mucinous PC, the correlation of PET/CT PCIs with surgery was very low (46). Nodules with a high proportion of mucin are not vascularized and are characterized by deficient metabolic activity and relatively lower presence of tumor cells.
Moreover, most mucinous PC is pseudomyxoma peritonei from low-grade appendiceal tumors; therefore, they have an intrinsically lower metabolism (47). Due to its ability to evaluate soft tissue alterations, MRI has been proposed as a complementary method for the assessment of tumoral involvement of the mesentery and small bowel. Klumpp et al. have reported excellent results for MRI, attributable to its superior soft-tissue contrast and capability to provide additional information about tissue characteristics through dynamic contrast-enhanced imaging, thus aiding the differentiation between malignant and other tissues (48). MRI also offers high sensitivity for liver metastasis and small perihepatic peritoneal implants owing to its superior soft-tissue contrast (48). MRI with diffusion-weighted imaging (DWI) in patients with PC from CRC, with a sensitivity of 88%, specificity of 74% in all tumor sizes and strong correlation with PCI scores calculated intraoperatively (49).
The gold standard for evaluating metachronous PC is diagnostic laparoscopy, which can also be helpful in avoiding unnecessary laparotomy in patients with pervasive disease. It is particularly useful to evaluate the involvement of small bowel, mesentery, and the PCI score, which can avoid unnecessary further surgery (50). However, at the Fifth International Workshop on Peritoneal Surface Malignancy, only 10% experts considered laparoscopy a fundamental modality in the preoperative evaluation of patients with PC, whereas 78% considered it a useful but not a fundamental method (28). This may be due to various factors that may cause incomplete assessment such as adhesions due to previous surgery or hostile abdomen due to extensive peritoneal disease and port site recurrence (28,50). Moreover, disadvantages of staging laparoscopy include inability to accurately assess tumor involvement of the retroperitoneal structures such as a pancreas, ureter, and omental bursa across to the celiac axis (51,52). Therefore a recent review recommended diagnostic laparoscopy in conjunction with routine imaging modalities to select patients who would most benefit from CRS and HIPEC (53).
Quantitative indicators by numeric score
Quantitative prognostic indicators for PC are essential in the management of peritoneal surface malignancy. They provide a specific language to quantify tumor burden from the standpoint of prognosis and the suitability of CRS. Various institutions have developed patient selection criteria for CRS with HIPEC based on tumor implant size and burden within the peritoneal cavity. An increased tumor burden is a negative prognostic factor and the use of quantitative prognostic indicators allows a more precise prediction of treatment outcomes (8).
PCI score
The PCI is an assessment tool that combines lesion size (LS-0 to LS-3) and tumor distribution in 13 abdominopelvic regions (AR-0 to AR-12) to quantify extent of disease as a numerical score (PCI-0 to PCI-39) (54). Many studies have used this system to demonstrate marked survival advantages of low PCI score (3,55-58). Sugarbaker et al. reported that large implant size and wide distribution of peritoneal implants to multiple sites within the abdomen and pelvis carry a uniformly poor prognosis (3). Ellisa et al. reported that the peritoneal index, based on an arbitrary cutoff of 15, had a significant impact on survival, with three-year survival rates of 60.3% and 32.5% among patients with values below and above this cutoff, respectively (55). They also reported that a PCI score ≤19 was a positive independent prognostic factor for overall survival (OS) (59). Kecmanovic et al. reported that the median survival time of patients with PCI ≤13 was 16.8 months, significantly higher than that of 6.9 months in patients with PCI >13 (57). Yan et al. reported that a PCI score ≤13 was associated with improved survival (versus >13, P=0.016) (58). Goéré et al. reported that the OS did not differ significantly between curative and palliative patients when the PCI score was >17 (56). However, there are some caveats in the use of the PCI score for PC from CRC. Currently, the cutoff for a ‘low’ PCI score associated with a favorable survival has not been clearly defined, in that the available clinical evidence does not allow a reliable prediction of when it is suitable to proceed with combined treatment based solely on the PCI score (60).
Simplified peritoneal cancer index (SPCI)
The Dutch SPCI registers the presence versus the absence of tumors in seven abdominal areas: left and right subdiaphragmatic spaces, subhepatic space, omentum, transverse colon, small intestine/mesentery, ileocolic region, and pelvis. This system is used by the Netherlands Cancer Institute for prognostic assessment. Verwaal et al. reported that the important factors predicting survival were number of affected regions, SPCI, and completeness of cytoreduction (CCR) score (40). SPCI has the advantage of easy evaluation and recording of anatomically separated lesions compared to the PCI.
CCR score
Jacquet et al. and Sugarbaker et al. proposed a CCR score that quantifies the extent of residual disease into four categories. CCR-0 indicates no visible evidence of residual tumor, CCR-1 indicates residual tumors ≤2.5 mm in diameter, CCR-2 indicates residual tumors between 2.5 mm and 2.5 cm, and CCR-3 indicates residual tumors >2.5 cm or a confluence of disease present at any site (54). In the registry study by Glehen et al., the overall median survival was 19.2 months. Patients who completed CRS had a median survival of 32.4 months, compared to 8.4 months in patients who could not complete CRS. The positive independent prognostic indicator by multivariate analysis was complete cytoreduction (61). Verwaal et al. reported that cytoreduction and HIPEC could result in long-term survival in patients with PC of colorectal origin (62).
Peritoneal surface disease severity score (PSDSS)
The PSDSS is an integral index that scores degree of clinical symptoms, extent of intraperitoneal metastasis, and morphology of tumor. PSDSS is evaluated using the original algorithm based on differentiating OC patients into two pathogenetic types depending on their histological and immunohistochemical findings. The PSDSS stages are scored from I to IV based on the scores for each of three clinicopathological parameters (stage I: ≤3 points; stage II: 4–6 points; stage III: 7–10 points, and stage IV: ≥11 points).
Yarema et al. reported an OS time for patients with PSDSS stage I, II, III, and IV ovarian cancer, of 48±25.3, 26.5±4.7, 15.5±4, and 6±4.3 months, respectively. Multivariate analysis showed that PSDSS stage was the only independent predictor of survival (63). However, in a study comparing PCI score and PSDSS in patients with colorectal PC, Ng et al. reported that the PCI score alone was a significant prognostic factor for OS and disease-free survival (DFS) in univariate and multivariate analysis (64).
Colorectal-PC (COREP) score
The COREP score was developed using HRs from histology, hematological status, serial STMs, and STM changes over time. The COREP score predicts low cancer-specific survival (12 months) and has a high sensitivity (80%) and specificity (100%) (16). Peter et al. reported that the COREP score was better than PSDSS and PS at predicting survival rate. Moreover, COREP (≥6) score was more accurate than PCI (>20) score for predicting poor prognosis (65).
Colorectal peritoneal metastases prognostic surgical score (COMPASS)
COMPASS is a prognostic pre-cytoreduction nomogram consisting of four parameters that are prognostically relevant for OS: age, PCI score, locoregional lymph node status, and signet ring cell histology. Peter reported that COMPASS was more accurate than PSDSS in predicting survival of patients undergoing CRS with HIPEC. It can be used to assist decisions regarding continuing cytoreduction and HIPEC and can provide valuable information in the follow-up period after CRS with HIPEC (66).
Techniques, chemotherapy agents, and safety of CRS
CRS and HIPEC techniques
The principle of CRS is to completely remove macroscopic tumors or leave a small residual tumor volume <2.5 mm, which is sufficient for the therapeutic effect of HIPEC (32). The CRS and peritonectomy procedures have been described and standardized by Sugarbaker (25). Sugarbaker described six surgical procedures for peritonectomy, including pelvic peritonectomy, greater omentectomy, left subphrenic peritonectomy, right subphrenic peritonectomy, lesser omentectomy, and associated visceral resection (stomach, small bowel, etc.). However, with an increasing number of treatment centers, there are differences in CRS techniques among surgeons. Kusamura et al. (67) reported the technical aspect of CRS, which is a consensus statement on the management of peritoneal surface malignancy, in 2006. The radicality of the peritonectomy procedure, timing of bowel anastomoses with HIPEC, indications of protective ostomies, and cytoreduction of neoplastic nodules <2.5 mm were the controversial issues in surgical technique. The consensus categorized peritoneal malignancy according to the pattern of spread. In the case of restricted peritoneal metastasis from CRC, most surgeons voted that partial peritonectomy is sufficient in intestinal-type CRC. Partial peritonectomy was considered sufficient in mucinous-type CRC by 70% of surgeons. Characteristics of pseudomyxoma peritonei differ from those of CRC and around 50% surgeons agreed that complete peritonectomy is necessary.
Bowel anastomosis timing related to HIPEC varies greatly among surgeons. The impact of hyperthermia on top of chemotherapy on anastomotic healing is unclear. Animal studies have found that anastomotic healing is impaired by mitomycin C (68,69). Generally, anastomosis before HIPEC reduces costs and operation time but impairs the integrity of the anastomosis (70). However, some surgeons have suggested that anastomosis after HIPEC is more difficult and could be hampered due to bowel edema after HIPEC (71).
Most surgeons perform electroevaporization for numerous small metastatic nodules (<2.5 mm) in the mesentery. The indications for ostomies are flexible according to surgeons and involved organs (71). The involvement of rectum is essential to make a stoma. If rectum can be preserved, a stoma can be avoided (72). Two studies have reported on bowel complications according to stoma formation, showing the same rates of bowel complications, although the stoma formation rates differed (10,73).
Heat has been shown to be cytotoxic in vitro at 42.5 °C (2). To reach this temperature in the abdominopelvic cavity, a specific device is required, which is a closed circuit enabling continuous hyperthermic chemoperfusion in the peritoneal cavity. Generally, two inflow outflow catheters each are placed in the abdominal cavity together with temperature probes and connected to a sterile closed circuit. The extracorporeal circuit pump, equipped with a reservoir filter, pumps heated perfusion isotonic fluid into abdominal cavity at a flow rate of 400–800 mL/min according to institutional protocol (74,75).
Intraperitoneal delivery techniques
HIPEC can be applied using open (Coliseum) and closed techniques. Open technique was described by Sugarbaker as follows: abdominal wall is suspended by a retractor frame and covered with a plastic sheet with a small slit that allows access to the abdomen for the manual stirring of the heated chemotherapeutic agents (76). Closed technique calls for application of HIPEC with the abdomen closed temporarily, or when all surgical processes have been completed. The closed technique aims to increase the penetration of the chemotherapeutic agents by utilizing a greater abdominal pressure than that of the open technique (75). Until now, no article has assessed the superiority of one technique over the other. Only one experimental study has been performed (77). Intraperitoneal hyperthermia can be achieved with both techniques. However, the systemic absorption and abdominal tissue penetration are better in open technique. In theory, the open technique may be more advantageous for more even drug and heat distribution. However, a clear conclusion has not yet been reached.
Safety consideration in CRS and HIPEC
CRS with HIPEC requires the use of cytotoxic drugs in the operation theatre where they not commonly used; thus, there is a risk of exposure to these drugs by the surgical team, anesthesiologists, the operating room staff.
In HIPEC, a heated chemotherapy solution is circulated in the peritoneal cavity using a roller pump and circuit, as described in the previous section (74,75). During this procedure, the abdomen is either closed or open (75,76). In the open technique, the surgeon manipulates the bowel loops to ensure more even distribution of the heat and chemotherapy; however, operating room personnel have an increased risk of exposure due to vaporization and direct contact with the drug as compared to the closed method (77). Exposure to cytotoxic agents has been shown to cause hair loss, headaches, acute irritation, and an increased rate of spontaneous abortions but not of congenital malformations and stillbirths (78-80). Therefore, all healthcare workers should wear protective disposable impermeable gowns (polyethylene-coated polypropylene) and shoe covers and eyewear for droplet protection (81). Double gloves should be worn by all staff at the surgical field and when cleaning up spills (81). Gloves should be changed every 30 min while continually working with cytotoxic agents as they do not entirely prevent cytotoxic drug penetration during prolonged contact (82). Surgeons in direct contact with a cytotoxic agent should wear outer gloves extending to the elbow (83).
Chemotherapy agents for HIPEC
Two main chemotherapy agents are administered to patients with PC from CRC: mitomycin C (10–50 mg/m2) over 60–120 min at 41–44 °C, and oxaliplatin (460 mg/m2) over 30 min at 42–44 °C preceded by an intravenous infusion of 4-FU (400 mg/m2) with leucovorin (20 mg/m2) regardless of intra-peritoneal delivery technique (Table 1). Superiority of MMC over oxaliplatin has been reported. Hompes et al. reported that MMC showed more hematologic-related events due to neutropenia (84). Rates of intra-abdominal complications did not differ significantly. Leung et al. showed that patients treated with HIPEC using oxaliplatin had better prognoses than those among patients administered MMC-based HIPEC (median survival: 54 vs. 26 months) (85). Conversely, Prada-Villaverde et al. reported that HIPEC with MMC might be superior to oxaliplatin-based HIPEC in patients with favorable histology or a low burden of PC (median survival: 54.3 vs. 30.4 months) (86). At present, no prospective study has compared these HIPEC regimens. Randomized controlled trials are necessary to standardize these chemotherapeutic regimens.
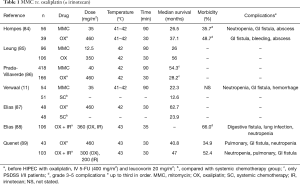
Full table
Before the advent of HIPEC with oxaliplatin, intravenous 5-FU and leucovorin maximized the effect of oxaliplatin (90). Elias et al. reported a median survival time of 63 months in patients administered HIPEC with oxaliplatin followed by intravenous 5-FU and leucovorin (87). Irinotecan is sometimes administered in combination with oxaliplatin. Elias et al. reported on short-term outcomes of intraperitoneal chemotherapy with oxaliplatin plus irinotecan (88). They concluded that irinotecan has a relatively high but acceptable incidence of adverse events. Quenet et al. evaluated the effect of addition of irinotecan. However, OS did not differ significantly between treatment groups with and without the addition of irinotecan (89). Various agents, including bevacizumab and H2O2, are used to increase the efficacy of HIPEC (91).
Outcomes of CRS and HIPEC
Short-term outcomes
CRS and HIPEC can cause considerable rates of morbidity and mortality, which requires long operation times and is technically challenging. Overall, the morbidity and mortality rates of HIPEC in recent studies in different centers ranged 12–52% and 0.9–5.8%, respectively (92). A recent meta-analysis and systemic review of 76 studies revealed mean morbidity and mortality of 33.0% and 2.8%, respectively, for CRS and HIPEC in patients with PC from CRC (93). The latest randomized phase III, multicenter trial reported an overall postoperative mortality rate of 1.5%, which did not differ significantly between CRS plus HIPEC and CRS alone. The morbidity rates did not differ statistically at 30 days. At 60 days, the major complication rate was significantly higher in patients that underwent HIPEC (24.1% vs. 13.6%, P=0.030).
The common causes of perioperative mortality are sepsis and multiorgan failure due to surgical complications. The most frequent complications include anastomotic leakage (0–9%), fistula (0–23%), intestinal perforation (0–10%), intraperitoneal sepsis (0–14%), abscess (0–37%), and ileus (0–86%) (94). We also evaluated the clinical outcomes of 102 patients who underwent CRS with HIPEC for appendiceal or CRC with PC in Yonsei between July 2014 and March 2016. The mean PCI score was 14.9±9.9 and HIPEC was performed by circulating a mixed solution of 35 mg/m2 mitomycin-C and 3 L of hypertonic solution (Dianeal, 1.5% dextrose peritoneal dialysis solution). Overall 30-day postoperative mortality rate was 0.0% and rate of short-term complications was 43.1%. Major complication rate was 18.6% (data not published).
The frequency of complications is also associated with the extent of disease. Casado-Adma et al. reported that PCI score (>30) was the only independent risk factor for gastrointestinal complications (95). Major complications are crucial because they may affect the oncologic outcome. Baratti et al. reported a five-year disease-specific survival rate of 14.3% in patients with PC from CRC who experienced major complications after CRS with HIPEC and 52.3% for those who did not. Five-year OS rates were 11.7% and 58.8% for patients who did and did not experience major complications, respectively. Multivariate analysis revealed that major morbidity was correlated to both worse overall and disease-specific survival (96).
Morbidity and morbidity are important in the aggressive treatment of patients with a disease that does not last long. In particular, major morbidity is also associated with survival.
To derive the maximal benefit of this treatment, careful patient selection with an optimal level of postoperative care must be advocated to avoid undesirable treatment complications (97).
Long-term outcomes
In well-selected patients with PC from CRC, CRS plus HIPEC with adjuvant chemotherapy may provide better oncologic outcomes compared to those in patients administered systemic chemotherapy alone.
A randomized Dutch phase III clinical trial demonstrated the efficacy of the procedure, reporting a better survival outcome for patients undergoing CRS and HIPEC in comparison with that in patients administered systemic chemotherapy alone (median survival: 22.3 vs. 12.6 months, P=0.032; corresponding to a two-year actuarial survival rate of 43% vs. 16%, P=0.014) (11). However, this first randomized trial for PC from CRC may not be generalized as the control arm of the study used systemic 5-FU alone and oxaliplatin was not included.
A recent meta-analysis also showed a significant difference in survival between CRS with HIPEC and systemic chemotherapy alone, suggesting that patients with PC from CRC could obtain more benefits from CRS with HIPEC than those from conventional treatment. The meta-analysis of 15 randomized controlled studies demonstrated that CRS with HIPEC as a comprehensive therapeutic strategy could bring a significant survival benefit for PC from CRC compared to that of palliative surgery alone or systemic chemotherapy [HR =2.67, 95% confidence interval (CI): 2.21–3.23, P<0.00001). Additionally, a summary analysis of these 76 studies showed that the median OS was about 29 months in the HIPEC group, which is significantly longer than the median OS of 17.9 months for patients with PC from CRC receiving contemporary chemotherapy (93). In a retrospective multicenter study of 294 patients with PC from CRC, Chua et al. reported a significant difference in survival between palliative treatment group (palliative surgery, systemic chemotherapy, supportive treatment) and curative (CRS with HIPEC) treatment groups (9 vs. 38 months) (98). The largest multicenter study including 506 patients from 28 institutions, reported overall 1-, 3-, and 5-year actuarial survival rates of 72%, 39%, and 19%, respectively. The overall 1-, 3-, and 5-year DFS rates were 40%, 16%, and 10%, respectively. They also reported that the extent of disease and completeness of CRS were independent prognostic indicators. The overall median survival time was 19.2 months. Patients with complete CRS had a median survival of 32.4 months, compared to 8.4 months in patients in whom complete CRS was not possible (P<.001). The 1-, 3-, and 5-year survival rates of patients with limited PC (PCI <13) were 92%, 50%, and 33%, respectively, and 62%, 22% and 11%, respectively, for patients with extended PC (PCI ≥13) (P<0.0001) (8). Our preliminary outcomes of 102 patients who underwent CRS with HIPEC for appendiceal or CRC cancer with PC in Yonsei were similar to those of previous studies. The overall median and median recurrence-free survival times were 20 and 11.5 months, respectively, during a median of 63.8 months of follow-up (data not published).
Associations between HIPEC regimens and oncologic outcome have not yet been sufficiently studied; however, there is reportedly no difference in survival rates between oxaliplatin and mitomycin in patients with PC from CRC. Huang reported that CRS with HIPEC significantly improved survival in both oxaliplatin and mitomycin groups, both showing similarly significant differences (93).
Studies have also assessed differences in long-term outcomes between HIPEC and early postoperative intraperitoneal chemotherapy (EPIC). In a prospective study comparing 98 patients with PC from CRC administered HIPEC + EPIC, HIPEC alone, and EPIC alone, recurrence-free survival of the HIPEC + EPIC group was significantly higher than those of the other two groups. There was no significant difference between the HIPEC alone and EPIC alone groups (98).
Recent trials and future perspectives
Following the recent negative results of randomized phase III, multicenter PRODIGE 7 trial, debates have arisen with regards to oncological outcome of CRS with HIPEC. The study aimed to evaluate the role of HIPEC after CRS. A total of 267 patients with histologically proven and isolated PC with PCI ≤25 were randomized (1:1) with complete macroscopic resection (R0/1 vs. R2), and neoadjuvant systemic chemotherapy. Patients were treated with CRS plus HIPEC with oxaliplatin or CRS alone, in association with systemic chemotherapy. After a median follow-up of 63.8 months, difference in OS was not statistically significant between the groups (OS: 41.7 m, 41.2 m, P=0.995/RFS: 13.1 m, 11.1 m, P=0.486), the addition of HIPEC with oxaliplatin did not influence survival benefit (99).
With the evolution of biologic agents, the synergism between target agents and HIPEC has been studied. The COMBATEC trial (27), with an enrollment of only 26 patients, was conducted to evaluate the feasibility, safety, and efficacy [defined as an improvement in progression-free survival (PFS)] of a multimodal treatment regimen consisting of pre and postoperative systemic combination chemotherapy and cetuximab in patients with complete cytoreduction with HIPEC (94). The study was terminated due to slow recruitment. The grade 3/4 morbidity rate was 44%, comparable to published data. The PFS was 14.9 months, similar to the results of other studies reporting PFS of 13–15 months.
Many recent clinical trials have evaluated prevention, especially second-look surgery and adjuvant HIPEC in patients at high risk of peritoneal metastasis (Table 2). Honore et al. reported that synchronous PM, synchronous isolated ovarian metastases, and a perforated primary tumor with serosa invasion and mucinous histological subtype to be the risk factors predictive of PC after curative surgery for CRC (104). Elias et al. proposed a new policy consisting of a systematic second-look surgery in patients at high risk of developing PM with resected minimal synchronous macroscopic, synchronous ovarian metastases, and perforation. A total of 41 patients without any sign of recurrence on imaging studies underwent second-look surgery for earlier and easier treatment of limited PC During second-look surgery, PC was identified and treated with CRS HIPEC in 23 of the 41 patients (56%). The five-year DFS and OS were 44% and 90%, respectively (105). These positive results prompted the design of a multicentric randomized trial (PROPHYLOCHIP). Patients with CRC at high risk of developing PC were randomized six months after adjuvant systemic chemotherapy to compare the results of second-look laparotomy and surveillance alone. The preliminary results failed to show a survival benefit of secondary-look surgery plus HIPEC in patients with PC from CRC (101). After a median follow-up of 51 months, the three-year DFS of 44% and 51% in the second-look and surveillance groups, respectively, did not differ significantly (P=0.75). In Italy, a currently recruiting randomized trial (NCT01628211) is investigating the role of second-look laparoscopy six months after radical resection of mucinous CRC. Preliminary results are expected in a few months. In cases of metachronous PM developing later (>12 months), PM can be missed by second-look surgery. The COLOPEC II (NCT03413254) trial was designed to evaluate the effectiveness of third-look laparoscopy as well as adjuvant HIPEC at the time of primary surgery. Other, similarly-designed studies are also evaluating the effectiveness of adjuvant HIPEC for patients with T4 disease or at high risk of PM such as perforation. The COLOPEC is a multicenter randomized controlled clinical trial. Patients with T4 perforated colon cancer without PM were randomized between patients administered simultaneous or staged (5–8 weeks) open or laparoscopic HIPEC followed by adjuvant systemic chemotherapy, and a control arm receiving systemic chemotherapy alone. The primary endpoint is peritoneal DFS at 18 months. Diagnostic laparoscopy will be performed routinely after 18 months postoperatively in both arms of the study in patients without evidence of disease based on routine follow-up using CT imaging and carcinoembryonic antigen (CEA) level. Patient recruiting has closed for this trial (102). The other studies are currently recruiting. The above-described studies on second-look surgery and adjuvant HIPEC highlight the fact that early treatment or prevention are the best strategies for the management of PM and will form the basis of future treatment for PM from CRC.
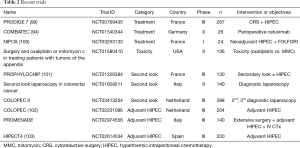
Full table
Conclusions
The distinctive nature of PC demands special attention to its diagnosis, prevention, multidisciplinary management, and palliative needs. CRS with HIPEC has driven a paradigm shift in the management of PC from a systemic to a locoregional approach. This complicated and aggressive treatment has become a standard treatment without level 1 evidence. Currently, trials with various study designs investigating CRS with HIPEC in PC from CRC are actively recruiting patients. The results of well-designed trials promise to expand the current indications for and utilization of CRS with HIPEC for patients with PC from CRC.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Hugen N, van de Velde CJ, de Wilt JH, et al. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann Oncol 2014;25:651-7. [Crossref] [PubMed]
- Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol 2004;5:219-28. [Crossref] [PubMed]
- Sugarbaker PH. Intraperitoneal chemotherapy and cytoreductive surgery for the prevention and treatment of peritoneal carcinomatosis and sarcomatosis.pdf. Semin Surg Oncol 1998;14:254-61. [Crossref] [PubMed]
- Van der Speeten K, Stuart OA, Sugarbaker PH. Pharmacokinetics and pharmacodynamics of perioperative cancer chemotherapy in peritoneal surface malignancy. Cancer J 2009;15:216-24. [Crossref] [PubMed]
- Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88:358-63. [Crossref] [PubMed]
- Yan TD, Cao CQ, Munkholm-Larsen S. A pharmacological review on intraperitoneal chemotherapy for peritoneal malignancy. World J Gastrointest Oncol 2010;2:109-16. [Crossref] [PubMed]
- Torres IJ, Litterst CL, Guarino AM. Transport of model compounds across the peritoneal membrane in the rat. Pharmacology 1978;17:330-40. [Crossref] [PubMed]
- Glehen O, Gilly FN. Quantitative prognostic indicators of peritoneal surface malignancy: carcinomatosis, sarcomatosis, and peritoneal mesothelioma. Surg Oncol Clin N Am 2003;12:649-71. [Crossref] [PubMed]
- Shen P, Hawksworth J, Lovato J, et al. Cytoreductive Surgery and Intraperitoneal Hyperthermic Chemotherapy With Mitomycin C for Peritoneal Carcinomatosis from Nonappendiceal Colorectal Carcinoma. Ann Surg Oncol 2004;11:178-86. [Crossref] [PubMed]
- Darwich AS, Ogungbenro K, Hatley OJ, et al. Role of pharmacokinetic modeling and simulation in precision dosing of anticancer drugs. Transl Cancer Res 2017;6:S1512-29. [Crossref]
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737-43. [Crossref] [PubMed]
- Meigs JV. Tumors of the female pelvic organs: Macmillan, 1934.
- Spratt JS, Adcock RA, Sherrill W, et al. Hyperthermic peritoneal perfusion system in canines. Cancer Res 1980;40:253-5. [PubMed]
- Spratt JS, Adcock RA, Muskovin M, et al. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res 1980;40:256-60. [PubMed]
- Sugarbaker PH. Surgical management of peritoneal carcinosis: diagnosis, prevention and treatment. Langenbecks Arch Chir 1988;373:189-96. [Crossref] [PubMed]
- Munnell EW. The changing prognosis and treatment in cancer of the ovary. A report of 235 patients with primary ovarian carcinoma 1952-1961. Am J Obstet Gynecol 1968;100:790-805. [Crossref] [PubMed]
- Griffiths CT, Parker LM, Fuller AF Jr. Role of cytoreductive surgical treatment in the management of advanced ovarian cancer. Cancer Treat Rep 1979;63:235-40. [PubMed]
- Long RT, Spratt JS Jr, Dowling E. Pseudomyxoma peritonei. New concepts in management with a report of seventeen patients. Am J Surg 1969;117:162-9. [Crossref] [PubMed]
- Ghosh BC, Huvos AG, Whiteley HW. Pseudomyxoma peritonei. Dis Colon Rectum 1972;15:420-5. [Crossref] [PubMed]
- Shingleton WW, Parker RT, Mahaley S. Abdominal Perfusion for Cancer Chemotherapy. Ann Surg 1960;152:583-91. [Crossref] [PubMed]
- Shingleton WW, Reeves JW Jr, Keppel RA, et al. Studies on abdominal organ perfusion for cancer chemotherapy. Ann Surg 1960;151:741-9. [Crossref] [PubMed]
- Dedrick RL, Myers CE, Bungay PM, et al. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep 1978;62:1-11. [PubMed]
- Palta JR. Design and testing of a therapeutic infusion filtration system. University of Missouri-Columbia, 1977.
- Fujimoto S, Shrestha RD, Kokubun M, et al. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding. Ann Surg 1988;208:36-41. [Crossref] [PubMed]
- Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29-42. [Crossref] [PubMed]
- Al-Shammaa HA, Li Y, Yonemura Y. Current status and future strategies of cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. World J Gastroenterol 2008;14:1159-66. [Crossref] [PubMed]
- Lemmens VE, Klaver YL, Verwaal VJ, et al. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer 2011;128:2717-25. [Crossref] [PubMed]
- Yan TD, Morris DL, Shigeki K, et al. Preoperative investigations in the management of peritoneal surface malignancy with cytoreductive surgery and perioperative intraperitoneal chemotherapy: Expert consensus statement. J Surg Oncol 2008;98:224-7. [Crossref] [PubMed]
- Koh JL, Yan TD, Glenn D, et al. Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol 2009;16:327-33. [Crossref] [PubMed]
- Thomassen I, van Gestel YR, Lemmens VE, et al. Incidence, prognosis, and treatment options for patients with synchronous peritoneal carcinomatosis and liver metastases from colorectal origin. Dis Colon Rectum 2013;56:1373-80. [Crossref] [PubMed]
- de Cuba EM, Kwakman R, Knol DL, et al. Cytoreductive surgery and HIPEC for peritoneal metastases combined with curative treatment of colorectal liver metastases: Systematic review of all literature and meta-analysis of observational studies. Cancer Treat Rev 2013;39:321-7. [Crossref] [PubMed]
- Bao P, Bartlett D. Surgical techniques in visceral resection and peritonectomy procedures. Cancer J 2009;15:204-11. [Crossref] [PubMed]
- Elias D, Benizri E, Pocard M, et al. Treatment of synchronous peritoneal carcinomatosis and liver metastases from colorectal cancer. Eur J Surg Oncol 2006;32:632-6. [Crossref] [PubMed]
- Lorimier G, Linot B, Paillocher N, et al. Curative cytoreductive surgery followed by hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis and synchronous resectable liver metastases arising from colorectal cancer. Eur J Surg Oncol 2017;43:150-8. [Crossref] [PubMed]
- Maggiori L, Goere D, Viana B, et al. Should patients with peritoneal carcinomatosis of colorectal origin with synchronous liver metastases be treated with a curative intent? A case-control study. Ann Surg 2013;258:116-21. [Crossref] [PubMed]
- Cloyd JM, Abdel-Misih S, Hays J, et al. Impact of Synchronous Liver Resection on the Perioperative Outcomes of Patients Undergoing CRS-HIPEC. J Gastrointest Surg 2018;22:1576-84. [Crossref] [PubMed]
- Razenberg LG, van Gestel YR, Lemmens VE, et al. The Prognostic Relevance of Histological Subtype in Patients With Peritoneal Metastases From Colorectal Cancer: A Nationwide Population-Based Study. Clin Colorectal Cancer 2015;14:e13-9. [Crossref] [PubMed]
- van Oudheusden TR, Braam HJ, Nienhuijs SW, et al. Poor outcome after cytoreductive surgery and HIPEC for colorectal peritoneal carcinomatosis with signet ring cell histology. J Surg Oncol 2015;111:237-42. [Crossref] [PubMed]
- Winer J, Zenati M, Ramalingam L, et al. Impact of aggressive histology and location of primary tumor on the efficacy of surgical therapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2014;21:1456-62. [Crossref] [PubMed]
- Verwaal VJ, van Tinteren H, van Ruth S, et al. Predicting the survival of patients with peritoneal carcinomatosis of colorectal origin treated by aggressive cytoreduction and hyperthermic intraperitoneal chemotherapy. Br J Surg 2004;91:739-46. [Crossref] [PubMed]
- Kwakman R, Schrama AM, van Olmen JP, et al. Clinicopathological Parameters in Patient Selection for Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Cancer Metastases: A Meta-analysis. Ann Surg 2016;263:1102-11. [Crossref] [PubMed]
- Pelz JO, Stojadinovic A, Nissan A, et al. Evaluation of a peritoneal surface disease severity score in patients with colon cancer with peritoneal carcinomatosis. J Surg Oncol 2009;99:9-15. [Crossref] [PubMed]
- de Bree E, Koops W, Kroger R, et al. Peritoneal carcinomatosis from colorectal or appendiceal origin: correlation of preoperative CT with intraoperative findings and evaluation of interobserver agreement. J Surg Oncol 2004;86:64-73. [Crossref] [PubMed]
- Suzuki A, Kawano T, Takahashi N, et al. Value of 18F-FDG PET in the detection of peritoneal carcinomatosis. Eur J Nucl Med Mol Imaging 2004;31:1413-20. [Crossref] [PubMed]
- Rakheja R, Makis W, Hickeson M. Extraovarian primary peritoneal carcinoma: staging with 18F-FDG PET/CT. Abdom Imaging 2012;37:304-8. [Crossref] [PubMed]
- Sommariva A, Evangelista L, Pintacuda G, et al. Diagnostic value of contrast-enhanced CT combined with 18-FDG PET in patients selected for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). Abdom Radiol (NY) 2018;43:1094-100. [Crossref] [PubMed]
- Passot G, Glehen O, Pellet O, et al. Pseudomyxoma peritonei: role of 18F-FDG PET in preoperative evaluation of pathological grade and potential for complete cytoreduction. Eur J Surg Oncol 2010;36:315-23. [Crossref] [PubMed]
- Klumpp BD, Schwenzer N, Aschoff P, et al. Preoperative assessment of peritoneal carcinomatosis: intraindividual comparison of 18F-FDG PET/CT and MRI. Abdom Imaging 2013;38:64-71. [Crossref] [PubMed]
- Low RN, Barone RM, Lee MJ. Surveillance MR imaging is superior to serum tumor markers for detecting early tumor recurrence in patients with appendiceal cancer treated with surgical cytoreduction and HIPEC. Ann Surg Oncol 2013;20:1074-81. [Crossref] [PubMed]
- Garofalo A, Valle M. Laparoscopy in the management of peritoneal carcinomatosis. Cancer J 2009;15:190-5. [Crossref] [PubMed]
- Iversen LH, Rasmussen PC, Laurberg S. Value of laparoscopy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Br J Surg 2013;100:285-92. [Crossref] [PubMed]
- Pomel C, Appleyard TL, Gouy S, et al. The role of laparoscopy to evaluate candidates for complete cytoreduction of peritoneal carcinomatosis and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol 2005;31:540-3. [Crossref] [PubMed]
- Seshadri RA, Hemanth Raj E. Diagnostic Laparoscopy in the Pre-operative Assessment of Patients Undergoing Cytoreductive Surgery and HIPEC for Peritoneal Surface Malignancies. Indian J Surg Oncol 2016;7:230-5. [Crossref] [PubMed]
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359-74. [Crossref] [PubMed]
- Elias D, Blot F, El Otmany A, et al. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer 2001;92:71-6. [Crossref] [PubMed]
- Goéré D. Extent of colorectal peritoneal carcinomatosis attempt to define a threshold above which HIPEC does not offer survival benefit a comparative study. Ann Surg Oncol 2015;22:2958-64. [Crossref] [PubMed]
- Kecmanovic DM, Pavlov MJ, Ceranic MS, et al. Treatment of peritoneal carcinomatosis from colorectal cancer by cytoreductive surgery and hyperthermic perioperative intraperitoneal chemotherapy. Eur J Surg Oncol 2005;31:147-52. [Crossref] [PubMed]
- Yan TD, Chu F, Links M, et al. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma: non-mucinous tumour associated with an improved survival. Eur J Surg Oncol 2006;32:1119-24. [Crossref] [PubMed]
- Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol 2010;28:63-8. [Crossref] [PubMed]
- Yan TD, Sim J, Morris DL. Selection of patients with colorectal peritoneal carcinomatosis for cytoreductive surgery and perioperative intraperitoneal chemotherapy. Ann Surg Oncol 2007;14:1807-17. [Crossref] [PubMed]
- Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004;22:3284-92. [Crossref] [PubMed]
- verwaal V. Long-Term Survival of Peritoneal Carcinomatosis of Colorectal Origin. Ann Surg 2005;12:65-71. [Crossref]
- Yarema R, Fetsych T, Volodko N, et al. Evaluation of the peritoneal surface disease severity score (PSDSS) in ovarian cancer patients undergoing cytoreductive surgery and HIPEC: Two pathogenetic types based study. J Surg Oncol 2018;117:1806-12. [Crossref] [PubMed]
- Ng JL, Ong WS, Chia CS, et al. Prognostic Relevance of the Peritoneal Surface Disease Severity Score Compared to the Peritoneal Cancer Index for Colorectal Peritoneal Carcinomatosis. Int J Surg Oncol 2016;2016:2495131. [Crossref] [PubMed]
- Cashin PH, Graf W, Nygren P, et al. Comparison of prognostic scores for patients with colorectal cancer peritoneal metastases treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2013;20:4183-9. [Crossref] [PubMed]
- Simkens GA, van Oudheusden TR, Nieboer D, et al. Development of a Prognostic Nomogram for Patients with Peritoneally Metastasized Colorectal Cancer Treated with Cytoreductive Surgery and HIPEC. Ann Surg Oncol 2016;23:4214-21. [Crossref] [PubMed]
- Kusamura S, O'Dwyer ST, Baratti D, et al. Technical aspects of cytoreductive surgery. J Surg Oncol 2008;98:232-6. [Crossref] [PubMed]
- Kuzu MA, Koksoy C, Kale T, et al. Experimental study of the effect of preoperative 5-fluorouracil on the integrity of colonic anastomoses. Br J Surg 1998;85:236-9. [Crossref] [PubMed]
- Shimizu T, Maeta M, Koga S. Influence of local hyperthermia on the healing of small intestinal anastomoses in the rat. Br J Surg 1991;78:57-9. [Crossref] [PubMed]
- Zanon C, Clara R, Bortolini M, et al. Chemohyperthermia for advanced abdominal malignancies: a new procedure with closed abdomen and previously performed anastomosis. Int J Hyperthermia 2001;17:456-64. [Crossref] [PubMed]
- Kusamura S, Baratti D, Virzi S, et al. Learning curve for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal surface malignancies: analysis of two centres. J Surg Oncol 2013;107:312-9. [Crossref] [PubMed]
- Moran BJ, Cecil TD. The etiology, clinical presentation, and management of pseudomyxoma peritonei. Surg Oncol Clin N Am 2003;12:585-603. [Crossref] [PubMed]
- Verwaal VJ, van Tinteren H, Ruth SV, et al. Toxicity of cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. J Surg Oncol 2004;85:61-7. [Crossref] [PubMed]
- Esquivel J. Technology of hyperthermic intraperitoneal chemotherapy in the United States, Europe, China, Japan, and Korea. Cancer J 2009;15:249-54. [Crossref] [PubMed]
- Glehen O, Cotte E, Kusamura S, et al. Hyperthermic intraperitoneal chemotherapy: nomenclature and modalities of perfusion. J Surg Oncol 2008;98:242-6. [Crossref] [PubMed]
- Stephens AD, Alderman R, Chang D, et al. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol 1999;6:790-6. [Crossref] [PubMed]
- Ortega-Deballon P, Facy O, Jambet S, et al. Which method to deliver hyperthermic intraperitoneal chemotherapy with oxaliplatin? An experimental comparison of open and closed techniques. Ann Surg Oncol 2010;17:1957-63. [Crossref] [PubMed]
- Valanis BG, Vollmer WM, Labuhn KT, et al. Association of antineoplastic drug handling with acute adverse effects in pharmacy personnel. Am J Hosp Pharm 1993;50:455-62. [PubMed]
- Valanis B, Vollmer WM, Steele P. Occupational exposure to antineoplastic agents: self-reported miscarriages and stillbirths among nurses and pharmacists. J Occup Environ Med 1999;41:632-8. [Crossref] [PubMed]
- Dranitsaris G, Johnston M, Poirier S, et al. Are health care providers who work with cancer drugs at an increased risk for toxic events? A systematic review and meta-analysis of the literature. J Oncol Pharm Pract 2005;11:69-78. [Crossref] [PubMed]
- Shida T, Takahashi S, Kobayashi S, et al. Assessment of the Risk of Anticancer Drug Permeability through Gowns Made of Various Materials. Iryo Yakugaku 2012;38:87-94.
- Stuart OA, Stephens AD, Welch L, et al. Safety monitoring of the coliseum technique for heated intraoperative intraperitoneal chemotherapy with mitomycin C. Ann Surg Oncol 2002;9:186-91. [Crossref] [PubMed]
- González-Bayón L, Gonzalez-Moreno S, Ortega-Perez G. Safety considerations for operating room personnel during hyperthermic intraoperative intraperitoneal chemotherapy perfusion. Eur J Surg Oncol 2006;32:619-24. [Crossref] [PubMed]
- Hompes D, D'Hoore A, Wolthuis A, et al. The use of Oxaliplatin or Mitomycin C in HIPEC treatment for peritoneal carcinomatosis from colorectal cancer: a comparative study. J Surg Oncol 2014;109:527-32. [Crossref] [PubMed]
- Leung V, Huo YR, Liauw W, et al. Oxaliplatin versus Mitomycin C for HIPEC in colorectal cancer peritoneal carcinomatosis. Eur J Surg Oncol 2017;43:144-9. [Crossref] [PubMed]
- Prada-Villaverde A, Esquivel J, Lowy AM, et al. The American Society of Peritoneal Surface Malignancies evaluation of HIPEC with Mitomycin C versus Oxaliplatin in 539 patients with colon cancer undergoing a complete cytoreductive surgery. J Surg Oncol 2014;110:779-85. [Crossref] [PubMed]
- Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 2009;27:681-5. [Crossref] [PubMed]
- Elias D, Goere D, Blot F, et al. Optimization of hyperthermic intraperitoneal chemotherapy with oxaliplatin plus irinotecan at 43 degrees C after compete cytoreductive surgery: mortality and morbidity in 106 consecutive patients. Ann Surg Oncol 2007;14:1818-24. [Crossref] [PubMed]
- Quenet F, Goere D, Mehta SS, et al. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann Surg 2011;254:294-301. [Crossref] [PubMed]
- Elias D, Bonnay M, Puizillou JM, et al. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: pharmacokinetics and tissue distribution. Ann Oncol 2002;13:267-72. [Crossref] [PubMed]
- Murono K, Kawai K, Hata K, et al. Regimens of Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis from Colorectal Cancer. Anticancer Res 2018;38:15-22. [PubMed]
- Roviello F, Caruso S, Marrelli D, et al. Treatment of peritoneal carcinomatosis with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: state of the art and future developments. Surg Oncol 2011;20:e38-54. [Crossref] [PubMed]
- Huang CQ, Min Y, Wang SY, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for peritoneal carcinomatosis from colorectal cancer: a systematic review and meta-analysis of current evidence. Oncotarget 2017;8:55657-83. [PubMed]
- Glockzin G, Zeman F, Croner RS, et al. Perioperative Systemic Chemotherapy, Cytoreductive Surgery, and Hyperthermic Intraperitoneal Chemotherapy in Patients With Colorectal Peritoneal Metastasis: Results of the Prospective Multicenter Phase 2 COMBATAC Trial. Clin Colorectal Cancer 2018;17:285-96. [Crossref] [PubMed]
- Casado-Adam A, Alderman R, Stuart OA, et al. Gastrointestinal complications in 147 consecutive patients with peritoneal surface malignancy treated by cytoreductive surgery and perioperative intraperitoneal chemotherapy. Int J Surg Oncol 2011;2011:468698. [Crossref] [PubMed]
- Baratti D, Kusamura S, Iusco D, et al. Postoperative complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy affect long-term outcome of patients with peritoneal metastases from colorectal cancer: a two-center study of 101 patients. Dis Colon Rectum 2014;57:858-68. [Crossref] [PubMed]
- Chua TC, Yan TD, Saxena A, et al. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: a systematic review of morbidity and mortality. Ann Surg 2009;249:900-7. [Crossref] [PubMed]
- Chua TC, Morris DL, Saxena A, et al. Influence of modern systemic therapies as adjunct to cytoreduction and perioperative intraperitoneal chemotherapy for patients with colorectal peritoneal carcinomatosis: a multicenter study. Ann Surg Oncol 2011;18:1560-7. [Crossref] [PubMed]
- Quenet F, Elias D, Roca L, et al. A UNICANCER phase III trial of hyperthermic intra-peritoneal chemotherapy (HIPEC) for colorectal peritoneal carcinomatosis (PC): PRODIGE 7. J Clin Oncol 2018;36:LBA3503. [Crossref]
- Sgarbura O, Samalin E, Carrere S, et al. Preoperative intraperitoneal oxaliplatin for unresectable peritoneal carcinomatosis of colorectal origin: a pilot study. Pleura Peritoneum 2016;1:209-15. [PubMed]
- Goere D, Glehen O, Quenet F, et al. Results of a randomized phase 3 study evaluating the potential benefit of a second-look surgery plus HIPEC in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP- NTC01226394). J Clin Oncol 2018;36:3531. [Crossref]
- Klaver CE, Musters GD, Bemelman WA, et al. Adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with colon cancer at high risk of peritoneal carcinomatosis; the COLOPEC randomized multicentre trial. BMC Cancer 2015;15:428. [Crossref] [PubMed]
- Arjona-Sánchez A, Barrios P, Boldo-Roda E, et al. HIPECT4: multicentre, randomized clinical trial to evaluate safety and efficacy of Hyperthermic intra-peritoneal chemotherapy (HIPEC) with Mitomycin C used during surgery for treatment of locally advanced colorectal carcinoma. BMC Cancer 2018;18:183. [Crossref] [PubMed]
- Honoré C, Goere D, Souadka A, et al. Definition of patients presenting a high risk of developing peritoneal carcinomatosis after curative surgery for colorectal cancer: a systematic review. Ann Surg Oncol 2013;20:183-92. [Crossref] [PubMed]
- Elias D, Honore C, Dumont F, et al. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann Surg 2011;254:289-93. [Crossref] [PubMed]


