Adjuvant radiotherapy in oesophageal cancer with positive circumferential resection margins—recurrence and survival outcomes
Introduction
The role of neoadjuvant and adjuvant therapies for oesophageal cancer has been widely investigated in recent years with a view to improve survival for locally advanced disease. Oesophageal cancer carries a poor prognosis with the majority of patients dying within a year of diagnosis, and only 15% surviving the disease beyond 5 years (1). Nevertheless, the 5-year survival rate has improved from 4.3% to 14.2%, from 1975–1979 to 1995–2000 (2). Results from the Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study (CROSS) demonstrated a significant survival advantage with neoadjuvant therapy (3).
Although the benefit of neoadjuvant therapy is clear and it is becoming the primary choice of treatment followed by surgical resection, the role of radiotherapy post-operatively has shown conflicting results, with several published studies, including a systematic review, showing no survival benefits (4-7). However, a recent large review demonstrated a statistically significant improvement in overall survival (OS) in patients with node positive or positive resection margin disease (8). Even with varying results, the UK national guidelines developed by the Upper Gastrointestinal Pathway Board Network Site Specific Group (NSSG), recommend that adjuvant radiotherapy should be considered in patients with locally advanced adenocarcinoma or R1 resection, however various issues such as regimens and timing need to be delineated (4).
The subset of patients suitable for adjuvant radiotherapy is still not defined. Studies have suggested an increased risk of negative long-term outcomes in patients with a positive circumferential resection margin (CRM) and various authors have suggested the use of adjuvant radiotherapy for those patients as a key to improving prognosis (8-15). Conversely, other authors have not confirmed the beneficial role of adjuvant radiotherapy for these patients (5-7,9,10,16-18), or present ambiguous findings (19). Furthermore, concerns have been raised with regards to the toxicity related to adjuvant radiotherapy (6,18).
The purpose of this study was to assess the impact of administration of adjuvant radiotherapy in patients with microscopically positive CRM following oesophageal resections for cancer with curative intent.
Methods
Patient data
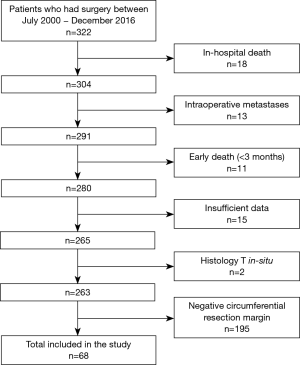
All consecutive patients who underwent surgery for resectable oesophageal or gastro-oesophageal junction (GOJ) carcinoma in a single centre between July 2000 and December 2016 were studied retrospectively. Patients who were found to have metastases intra-operatively, patients with in-hospital mortality or early death (defined as less than three months postoperatively) and patients with insufficient data such as circumferential margin measurements, use of adjuvant radiotherapy and oncological outcomes were excluded from the study (9,16). Overall three Consultant Upper Gastrointestinal surgeons were the primary operators, with an additional four surgeons performing six cases.
The parameters studied were age, sex, tumour location and type, grade of differentiation, pTNM staging, lymphovascular invasion, involvement of circumferential margin, length of tumour and involved to harvested lymph node ratio in the histology specimen, administration of neoadjuvant and adjuvant chemo-radiotherapy, performance status and American Society of Anaesthesiologists (ASA) score. The main outcomes studied were local recurrence, distant metastases, disease progression, progression-free survival (PFS) and OS. Local recurrence is defined as tumour recurrence at or near the resection site or involving nearby lymph nodes. Distant metastasis was defined as disease away from the primary site. Disease progression was defined as the diagnosis of either local recurrence or distant metastases in the follow up period. Tumour location was defined as upper, middle or lower third of the oesophagus, or GOJ tumours (20). Patients with significantly prolonged recovery, poor postoperative performance status or severe complications were not considered for adjuvant radiotherapy. Further reason for not administering adjuvant radiotherapy was patients’ informed decision.
Follow-up was calculated from the date of surgery until the last clinic appointment attended or death, whether this was cancer related or not. The hospital’s online medical notes system, pathology results and Picture Archiving and Communicating System (PACS) were used to assimilate the data onto a spreadsheet.
Pathological examination of resection specimen
Surgical resection was performed either as an open or laparoscopic procedure by a specialist upper gastrointestinal surgeon. The majority of cases were performed by a two-stage technique for Ivor-Lewis oesophagectomy, and other surgical approaches included McKewon oesophagectomy and total or extended total gastrectomy. All resected oesophageal specimens were fixed in formalin and analysed by a specialist gastro-intestinal pathologist. The circumferential margins were measured to the closest 0.1 mm and reported according to the UK Royal College of Pathologists (RCP) standard requirement for pathology reporting for oesophageal resection specimens, defining a circumferential margin as positive when the distance of tumour cells is less than 1 mm from the resection margin, or R1 margin (11,21). TNM staging was recorded according to the American Joint Committee on Cancer. Sixth edition TNM staging was used up to 2010, and 7th edition TNM staging used after 2010. The main difference was regarding the classification of loco-regional lymph node metastasis in relation to location (22).
PFS and OS
Local policy offers follow-up to patients post-operatively with three-monthly appointments in the first year, six-monthly in the second year, annually for the following 5 years and two-yearly thereafter. Further radiological imaging or endoscopy is considered if there is onset of new symptoms and clinical suspicion of recurrent disease. PFS was defined as the time interval between surgery and the last follow-up date or time of new diagnosis of recurrence or metastatic disease. Last follow-up was defined as the last clinic appointment or the last endoscopic or radiologic investigation confirming no evidence of cancer recurrence or metastatic disease. In this case end date was considered to be April 2017 for completion of data collection. For patients who had time interval greater than one year between their last follow-up and April 2017, PFS could not be defined as they could not be confidently considered disease-free without adequate follow-up and therefore, they were considered as lost to follow-up. OS was calculated up to April 2017 or date of death.
Statistical analysis
Bivariate correlations were assessed using Fisher’s exact test for dichotomous categorical variables and Log Rank for time-lines (PFS and OS). A P value of less than 0.05 was considered statistically significant. Two-tailed comparisons were consistently used where applicable. Statistical analysis was conducted using SPSS v23 (IBM, Armonk, NY, USA).
Results
Patient characteristics
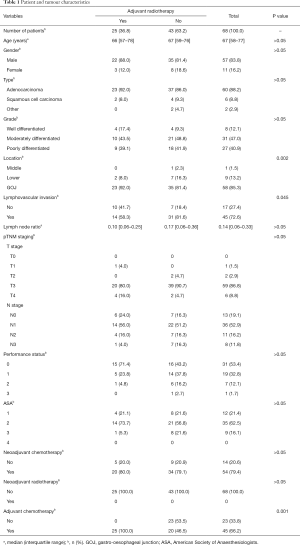
A total of 322 patients were initially considered for the study. Two hundred and sixty-three patients with oesophageal or GOJ cancer who underwent surgical resection with curative intent were assessed according to the set criteria (Figure 1). From the total number of patients, 68 patients (21.1%) had positive CRM on histological analysis according to the UK RCP definition and was the main focus of the study. This cohort had 57 male patients (83.8%) and 11 female patients (16.2%). The median age was 67 years with an interquartile range (IQR) from 58 to 77 years. Duration of follow-up for this cohort ranged from 2 to 121 months with a median follow-up of 13 months. Table 1 demonstrates the demographics and further characteristics for this cohort, including comparisons for those who received adjuvant radiotherapy and those who did not.
Full table
In all cases, neoadjuvant and adjuvant chemotherapy regimens varied throughout the years, regardless of inclusion in clinical trials. There was a predominance of neoadjuvant chemotherapy regimens including cisplatin, 5-fluorouracil (5-FU) or both agents in 91.6% of the recorded cases. Similarly, adjuvant chemotherapy regimens included either cisplatin, 5-FU or a combination of both in 83.5% of the recorded cases. Administration of neoadjuvant chemotherapy was not found to be statistically significantly correlated with PFS or OS. It was thus considered not necessary to exclude it as a confounding factor in further analysis.
Specimen characteristics
The resection specimens revealed the location of the tumour in the middle third oesophagus in one case and lower third in nine cases. Fifty-eight cases were classified as GOJ tumours. Histologically, there were 60 cases of adenocarcinomas, 6 cases of squamous cell carcinomas, and 2 cases of other subtypes. The median number of lymph nodes resected in each specimen were 20 with an IQR from 16 to 27. There was a median of 3 neoplastic lymph nodes confirmed histologically in each specimen (IQR: 1 to 6). Out of the 68 patients with positive circumferential margins, 25 patients received adjuvant radiotherapy as treatment for the involvement of their circumferential margins (36.8%). These patients received adjuvant chemotherapy too, except for one patient. Nineteen patients received chemotherapy concurrently, 4 patients sequentially and 1 patient had both concurrent and sequential chemotherapy.
Recurrence
Local recurrence was observed in 15 patients (22.1%) with 7 patients having received adjuvant radiotherapy and occurred more frequently at the anastomotic site (n=8). Other loco-regional disease recurrence included mediastinal lymph nodes (n=4), supraclavicular lymph nodes (n=2) and perigastric lymph nodes (n=1). Distant metastases were recorded in 42 patients (61.8%), 13 of who had received adjuvant radiotherapy. Common distant metastatic sites included lung (n=12), bone (n=8), peritoneal (n=8), liver (n=5) and brain (n=5). Other abdominal organs involved were the bowel and adrenal glands.
Adjuvant radiotherapy
Adjuvant radiotherapy regimen was delivered via an intensity modulated radiotherapy with 45–50 Gy in daily fractions of 1.8–2 Gy 5 days a week for 5 weeks. It was offered concurrently with chemotherapy (5-FU or capecitabine). The clinical target volume (CTV) included the tumour bed defined by the pre-operative CT scan, regional lymph nodes (perigastric, coeliac, paraaortic, splenic, hepatoduodenal, paracardial, paraoesophageal), and 2 cm beyond the proximal and distal resection margins.
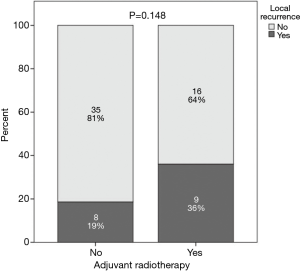
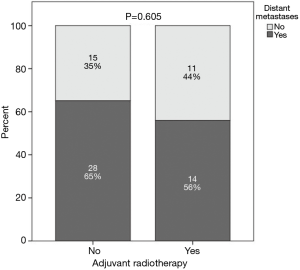
A comparison was performed between patients who had positive CRM and received adjuvant radiotherapy and those with positive CRM who did not receive adjuvant radiotherapy and this revealed no statistically significant difference between the 2 groups with regards to local recurrence (P=0.148), distant metastases (P=0.605), overall progression (P=0.561), PFS (P=0.663) or OS (P=0.538) (Figures 2-6).
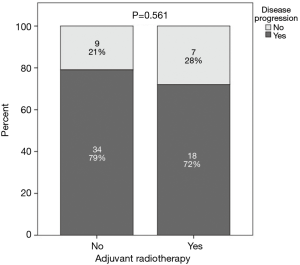
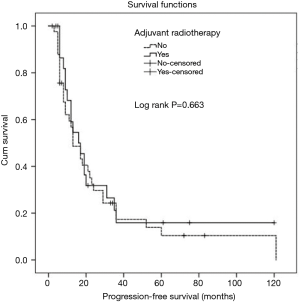
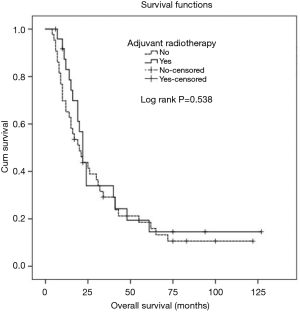
Comparison between both groups revealed three statistically significant parameters however none of these retained significance with multivariate analysis. Therefore, there was no effect on the overall findings of no correlation between adjuvant radiotherapy and outcomes (Table 1).
Discussion
This study demonstrates no benefit to oncological outcomes in patients with microscopically positive circumferential margins who have received adjuvant radiotherapy in locally advanced resectable oesophageal carcinoma, compared to those who did not receive adjuvant radiotherapy. There were no significant differences between the two groups with regards to local recurrence, distant metastases, overall progression, OS and PFS. This study adds to the literature base which presents conflicting evidence regarding the long-term benefits of adjuvant radiotherapy to this group of patients.
The present results are consistent with a study by Qui et al. who demonstrated no overall long-term survival advantage (18). Similarly, Ténière et al.’s study of 221 randomised patients showed control of local disease (30% with radiotherapy vs. 15% without), but no effects on survival (5). Interestingly, Fok et al.’s prospective trial of 130 patients demonstrated decreased loco-regional recurrence but also decreased survival which was attributed to high irradiation-related deaths and early metastatic recurrence (6). Furthermore, Song et al. failed to show any statistical significance in OS, PFS and loco-regional recurrence rates in patients who underwent post-operative radiotherapy for R1 resections following curative oesophagectomy for SCC, similarly to Park et al. and to the present study. Overall, a recent meta-analysis found no significant survival benefit with post-operative radiotherapy (P=0.11) (9,15,16).
Multiple studies have demonstrated a partial positive impact on survival and reduced incidence of distant metastases, but not loco-regional recurrence in patients having adjuvant radiotherapy for positive margins (10,19). Furthermore, it is already known that R1 groups are associated with positive lymph nodes and studies have shown a significant relationship between positive lymph nodes and adjuvant radiotherapy in regard to overall 5-year survival, compared to those having surgery alone (18% vs. 34%; P=0.038) (23,24). A large study using the National Cancer Data Base showed a significant improved 3-year OS in patients with localised disease receiving adjuvant radiotherapy with positive margins compared to those receiving surgery alone (36.4% vs. 18%; P<0.001) (8). There was no effect on survival in patients with negative margins having received radiotherapy compared to those who did not (P=0.24) (8). O’Neill’s study evaluated the significance of adjuvant therapy in positive margin patients. There was no significant difference in survival benefit between patients with CRM of 0 mm and patients with CRM of 0.1–0.9 mm. However, both groups had a significantly poorer survival outcome when compared to patients with at least 1 mm clear margin. In this group, radiotherapy conferred a better outcome, with an improvement from 18.6 to 28.6 months longer survival (P=0.009) (11). Yu et al. assessed the benefit of extended volume external beam radiotherapy on high risk oesophagectomy patients, including those with positive margins. There was a significant benefit with local-regional recurrence at the anastomotic site (P=0.003) (25).
Currently, adjuvant radiotherapy is accepted in clinical practice for patients with positive CRM although there are no established indications and the findings are conflicting. Discrepancies noted between older studies and recent ones may be attributable to different radiation doses/techniques used and differences in neoadjuvant treatments which limit comparison between studies (8). The current study did not show any benefit regarding local recurrence, distant metastases, OS and PFS, however, the role of adjuvant radiotherapy for patients with positive CRM needs further critical evaluation, in the context of all different treatment modalities in a multidisciplinary setting, considering also possible radiotherapy-related increased morbidity.
There are limitations in this study due to its retrospective nature. Chemoradiation regimes have not been uniform through the years, which may affect outcomes. Toxicity from adjuvant radiotherapy regimes was not studied and this could have added more information to the real benefit of this treatment modality. Furthermore, the small sample size might be responsible for detecting no statistically significant correlations. However, this is one of the largest published cohorts, and in order to obtain a larger sample size, multi-centre cohorts would be required. Finally, although consecutive patients were included, there may have been a selection bias for patients who were offered adjuvant radiotherapy based on their fitness, performance status and other parameters. However, this selection reflects current clinical practice.
Conclusions
There is significant uncertainty about the long-term effects of adjuvant treatment and specifically adjuvant radiotherapy. The positivity of the CRM is an established risk factor for poorer oncological outcomes, however there is conflicting evidence in literature supporting the benefits of adjuvant radiotherapy in these patients, with the majority limited to retrospective and non-randomised studies. The present study confers no advantage of administering adjuvant radiotherapy in patients with positive CRM, in regard to local recurrence, distant metastases, total progression, PFS or OS. However, multi centre prospective randomised studies are needed for further evaluation of this relation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Approval was not sought by an institutional review board for this single centre study. It has had local approval by the institution research and audit department. The study outcomes will add to the current literature base to help inform future management of these patients. Data was retrieved from hospital medical records and therefore informed consent was not required. The patients’ personal data was anonymised and kept securely within the relevant hospital department on the secure password protected computers.
References
- Oesophageal cancer statistics. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/oesophageal-cancer#heading-Two
- Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975-2001, with a special feature regarding survival. Cancer 2004;101:3-27. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJB, Hulshof MCCM, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Upper GI (Oesophagogastric) Pathway Board (NSSG) Guidelines; London Cancer North and East; 2013. Available online: http://www.londoncancer.org/media/85393/london-cancer-ugi-og-guidelines-v01-final-20140106.pdf
- Ténière P, Hay JM, Fingerhut A, et al. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicentered controlled trial. French University Association for Surgical Research. Surg Gynecol Obstet 1991;173:123-30. [PubMed]
- Fok M, Sham JS, Choy D, et al. Postoperative radiotherapy for carcinoma of the esophagus: a prospective, randomized controlled study. Surgery 1993;113:138-47. [PubMed]
- Xiao ZF, Yang ZY, Liang J, et al. Value of radiotherapy after radical surgery for esophageal carcinoma: a report of 495 patients. Ann Thorac Surg 2003;75:331-6. [Crossref] [PubMed]
- Wong AT, Shao M, Rineer J, et al. The Impact of Adjuvant Postoperative Radiation Therapy and Chemotherapy on Survival After Esophagectomy for Esophageal Carcinoma. Ann Surg 2017;265:1146-51. [Crossref] [PubMed]
- Park HJ, Kim HJ, Chie EK, et al. The influence of circumferential resection margin status on loco-regional recurrence in esophageal squamous cell carcinoma. J Surg Oncol 2013;107:762-6. [Crossref] [PubMed]
- Gilbert S, Martel AB, Seely AJ, et al. Prognostic significance of a positive radial margin after esophageal cancer resection. J Thorac Cardiovasc Surg 2015;149:548-55. [Crossref] [PubMed]
- O’Neill JR, Stephens NA, Save V, et al. Defining a positive circumferential resection margin in oesophageal cancer and its implications for adjuvant treatment. Br J Surg 2013;100:1055-63. [Crossref] [PubMed]
- Wu J, Chen QX, Teng LS, et al. Prognostic significance of positive circumferential resection margin in esophageal cancer: a systematic review and meta-analysis. Ann Thorac Surg 2014;97:446-53. [Crossref] [PubMed]
- Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113: a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for oesophageal cancer. J Clin Oncol 2007;25:3719-25. [Crossref] [PubMed]
- Chen J, Zhu J, Pan J, et al. Postoperative radiotherapy improved survival of poor prognostic squamous cell carcinoma oesophagus. Ann Thorac Surg 2010;90:435-42. [Crossref] [PubMed]
- Song S, Chie EK, Kim HJ, et al. Role of postoperative radiotherapy for microscopic margin involvement in the squamous cell carcinoma of esophagus. Cancer Res Treat 2013;45:202-9. [Crossref] [PubMed]
- Malthaner RA, Wong RK, Rumble RB, et al. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: a systematic review and meta-analysis. BMC Med 2004;2:35. [Crossref] [PubMed]
- Zieren HU, Muller JM, Jacobi CA, et al. Adjuvant postoperative radiation therapy after curative resection of squamous cell carcinoma of the thoracic oesophagus: a prospective randomised study. World J Surg 1995;19:444-9. [Crossref] [PubMed]
- Qiu B, Li J, Wang B, et al. Adjuvant Therapy for a Microscopically Incomplete Resection Margin after an Esophagectomy for Esophageal Squamous Cell Carcinoma. J Cancer 2017;8:249-57. [Crossref] [PubMed]
- Markar SR, Gronnier C, Duhamnel A, et al. Significance of Microscopically Incomplete Resection Margin After Esophagectomy for Esophageal Cancer. Ann Surg 2016;263:712-8. [Crossref] [PubMed]
- Siewert JR, Stein HJ. Carcinoma of the gastroesophageal junction - classification, pathology and extent of resection. Dis Esophagus 1996;9:173-82.
- Chan DS, Reid TD, Howell I, et al. Systematic review and meta-analysis of the influence of circumferential resection margin involvement on survival in patients with operable oesophageal cancer. Br J Surg 2013;100:456-64. [Crossref] [PubMed]
- AJCC. Available online: https://cancerstaging.org/Pages/default.aspx
- Xiao ZF, Yang ZY, Miao YJ, et al. Influence of number of metastatic lymph nodes on survival of curative resected thoracic esophageal cancer patients and value of radiotherapy: a report of 549 cases. Int J Radiat Oncol Biol Phys 2005;62:82-90. [Crossref] [PubMed]
- Jabbour SK, Thomas CR Jr. Radiation therapy in the postoperative management of esophageal cancer. J Gastrointest Oncol 2010;1:102-11. [PubMed]
- Yu E, Dar R, Rodrigues GB, et al. Is extended volume external beam radiation therapy covering the anastomotic site beneficial in post-esophagectomy high risk patients? Radiother Oncol 2004;73:141-8. [Crossref] [PubMed]