Percutaneous radiofrequency versus microwave ablation for management of hepatocellular carcinoma: a randomized controlled trial
Introduction
Hepatocellular carcinoma (HCC) is one of aggressive tumors that usually arise on top of background of liver cirrhosis (1). It is the fifth most common cancer in the world (2). Also, it is the third most common cause of cancer related deaths (1). In Egypt, it is the most common malignancy among males, comprising 33% of all cancer cases and the second most common malignancy in females, coming after breast cancer, comprising 13% of all cancer cases (3). The optimal management of HCC depends on many factors including tumor size, number, distribution, the relationship of the tumor to hepatic vasculature, presence or absence of lymph nodes or distant metastases, the Child-Pugh score, the functional status of the patient, the suitability for liver transplantation and local expertise (3).
Many available treatment modalities for HCC are present including liver resection, liver transplantation, radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), microwave ablation (MWA), transarterial chemoembolization (TACE), yttrium ablation, conformal radiation therapy and systemic therapy (e.g., sorafenib) (4,5).
RFA depends on ultrasound guided delivery of high frequency current to the targeted tissue via a needle electrode (6,7).
RFA is more effective against small tumors less than 3 cm. It is difficult to destroy lesions above 5 cm using the currently available needles (6-8). A margin of 0.5–1.0 cm of non-malignant liver tissue should be ablated to ensure treatment of the peripheral tumor that includes any microscopic extension beyond the radiologically visible margins (7).
Presence of the tumor nearby a large blood vessel decreases the efficacy of RFA by the heat sink effect when thermal energy escapes from the targeted tissue to the vessels adjacent to it (9,10). Also, Tissue charring acts as electrical insulators and limit the effect of RFA through increased impedance. As a result, the size and shape of the ablation zone may be unpredictable and multiple sessions may be necessary for complete tumor ablation (9,11).
Compared to RFA, MWA is another local ablation method that is increasingly used. It depends on delivering high frequency microwave into the tumor tissue creating electromagnetic energy leading to rapid directional changes in the current causing water dipoles to oscillate with subsequent heat generation that leads to coagulative necrosis of the tumor cells (6,7).
Unlike RFA, the MWA is less affected by the heat-sink effect and increased impedance of the ablated tissue, and so the shape and size of ablated zone created by MWA is more predictable (9,11). Also, during MWA simultaneous multi-probe activation can be performed, which is not possible with RFA because of the potential electrical interference (7).
Despite the theoretical advantages of MWA over RFA, MWA still not included in the standard guidelines for HCC management. There are scarce studies comparing results of both techniques in the real life, most of which are retrospective, so our aim was to compare efficacy of RFA and MWA in management of HCC using a prospective randomized controlled trial.
Methods
Subjects and study design
All patients with definite HCC on top of liver cirrhosis related to HCV who were referred to Alexandria University Hepatobiliary Unit during the 6-month period from 15/6/2017 to 15/12/2018 whose HCC lesions are 3 or less with no lesion more than 5 cm and no vascular invasion or extrahepatic spread were enrolled to the study, with exclusion of those with positive HBsAg, history of alcohol consumption, patients with other known causes of chronic liver disease, patients who have received previous DAAs for HCV and patients who have received previous locoregional treatment for HCC. Patients were randomly assigned to either RFA or MWA. The study was conducted in accordance with the provisions of the Declaration of Helsinki, as revised in 2013, and Good Clinical Practice guidelines. It was approved by the Ethics Committee of Faculty of Medicine, Alexandria University (IRB No. 00007555). An informed consent was obtained from all subjects included in the study.
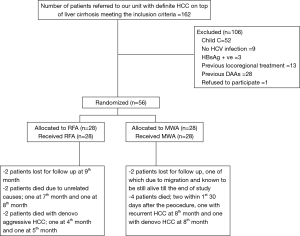
The CONSORT flow diagram of the study is shown in Figure 1.
Procedures
All patients included in the study were evaluated as regards Clinical Evaluation, Laboratory Investigations including complete blood picture (CBC), serum aspartate and alanine aminotransferases (AST and ALT), serum albumin, serum bilirubin, serum, alkaline phosphatase, prothrombin activity, INR, alfa fetoprotein (AFP), HCV antibodies, hepatitis B surface antigen and hepatitis B core antibody using enzyme-linked immune-sorbent assay (ELISA), HCV RNA levels in serum using real time polymerase chain reaction assay and HCV genotyping +/− subtyping if indicated. Liver disease severity was assessed based on modified Child Pugh classification (CTP) and model for end-stage liver disease.
Radiological evaluation depended on a recent Triphasic CT abdomen and/or dynamic MRI performed within 4 weeks before ablation for diagnosis of HCC based on characteristic enhancement pattern and to determine number, size and site of tumors and to exclude portal vein invasions. Hepatic lesions were classified according to LI-RADS classification (12). Definite HCC (LR-5) lesions were the only to be considered for inclusion in the study.
All included patients were randomized for HCC ablation using RFA or MWA. If multiple lesions were present, all were treated with the same method. For RFA (Angiodynamics RITA model 1,500×, USA) generator and RITA StarBurst XL needle were used complying with manufacturer’s instructions. For MWA, a 14 gauge 200 mm disposable MWA probe (AMICA probe MW) and a 2.45 GHz generator (AMICA GEN AGN-H-1.2, Italy) were used. Duration and wattage used for ablation were chosen according to the manufacturer’s instructions.
Local response was assessed by triphasic CT done 4 weeks after the treatment. Those with residual activity were retreated by RFA or MWA according to the initial randomization. An extra follow up triphasic CT was performed after another 4 weeks.
All patients were followed every 3 months after the procedure to discover any HCC recurrence using triphasic CT. Response was evaluated according to modified RECIST criteria (13). Also, modified Child-Pugh score was evaluated on the same intervals.
Statistical analysis of the data
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0 (Armonk, NY: IBM Corp). Qualitative data were described using number and percent. The Kolmogorov-Smirnov test was used to verify the normality of distribution. Quantitative data were described using range (minimum and maximum), mean, standard deviation and median. Significance of the obtained results was judged at the 5% level. Chi-square test was used for categorical variables, to compare between different groups. Fisher’s Exact or Monte Carlo correction for chi-square were used when more than 20% of the cells have expected count less than 5. Student t-test was used for normally distributed quantitative variables, to compare between two studied groups. Mann Whitney test was used for non-normally distributed quantitative variables, to compare between two studied groups. Kaplan-Meier Survival curve was used and cox regression was done for the significant relation with overall survival.
Results
There were no statistically significant differences between both groups regarding age and sex. Men were predominating in both groups (78.6% in the RFA group and 75% in the MWA group), whereas the mean age was nearly 55 years in both groups, ranging from 42 to 80 years.
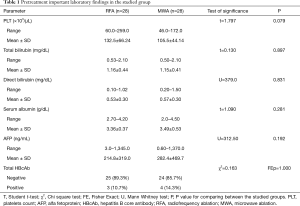
There were no statistically significant differences between both groups regarding the pre-treatment laboratory findings including platelet counts, albumin, total bilirubin and AFP levels (Table 1). Also, there was no difference regarding the pre-treatment Child-Pugh score. Twenty-two patients were Child-Pugh class A vs. 6 patients were Child-Pugh class B in both groups. The mean Child-Pugh score was 5.86 for the RFA group vs. 5.79 for the MWA group (P=0.778). Performance status and MELD score were not significantly statistically different between both groups before HCC ablation (Table 2).
Full table

Full table
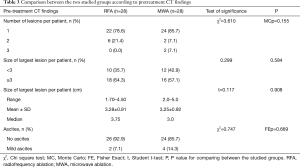
In addition, there were no statistically significant differences between both groups regarding number and sizes of HCC lesions. Twenty-two patients in the RFA group had single lesion while 6 patients had 2 lesions. In the MWA group, 24 patients had single lesion, while 2 patients had 2 lesions and the other 2 had 3 lesions (P=0.155). Eighteen patients in the RFA group had lesions that measured 3 cm or more vs. 16 patients in the MWA group (P=0.584). The mean size of largest lesions per patient in the RFA group was 3.28 vs. 3.25 in the MWA group (P=0.908) (Table 3).
Full table
There was no statistically significant difference between both groups regarding number of sessions needed till complete ablation of the tumor, but the duration was significantly shorter in the MWA group. The mean ablation time at RFA group was 14.21 minutes while it was 4.41 minutes at the MWA group (P<0.001) (Table 4).

Full table
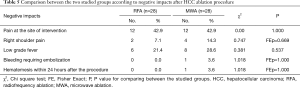
Regarding complications after the procedure, there were no clinically significant differences between both groups. Two major complications occurred after MWA, namely bleeding from the tumor in one patient and hematemesis in the next day of the procedure in another patient, but this was not statistically significant (Table 5).
Full table
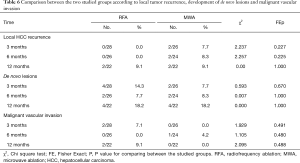
Local tumor recurrence didn’t differ statistically between both groups. At 6 months the local recurrence was 0% at the RFA group while it was 8.3% at the MWA group (P=0.225). At 12 months, the local recurrence was 9.1% in both groups (Table 6).
Full table
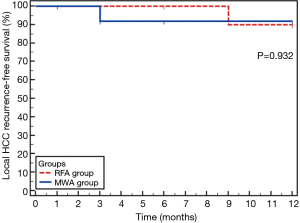
Kaplan-Meier estimates of local tumor recurrence-free survival at 1-year follow up was 90.9% of subjects for the RFA group and 92.3% for the MWA group which was not statistically significant difference (Figure 2). The estimated mean local recurrence free time was 11.7 months in the RFA group and 11.3 months in the MWA group.
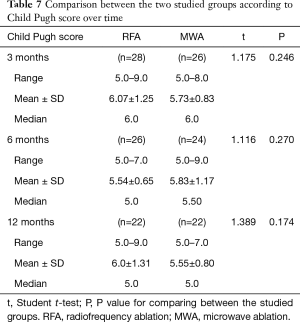
Till the end of 1-year follow up, there were no statistically significant differences between both groups regarding the development of de novo lesions or macrovascular tumor invasion (Table 6). In addition, there was no statistically significant difference between both groups regarding Child-Pugh score over time (Table 7).
Full table
Two patients died within 30 days after MWA while no one died after RFA during the same period, but this was not statistically significant difference. By the end of 1-year follow up, four patients died in each group (Table 8). Two patients in the RFA group died with unrelated causes (one with pulmonary embolism and the other due to intracerebral hemorrhage). The other two died with de novo aggressive HCC. The two early mortalities in MWA group were due to liver decompensation, one of them died few days after successfully stopped bleeding from the tumor occurred on the same day of the procedure. Another patient died with recurrent HCC while the fourth one died with de novo HCC.

Full table
Discussion
Although the theoretical advantages of MWA over RFA, results of clinical studies are different. Most of these studies are retrospective.
Our study was a randomized controlled trial. This is similar to Shibata et al. (14) and Violi et al. (15). On the other hand, Lu et al. (16), Ohmoto et al. (17), Ding et al. (18), Zhang et al. (19), Abdelaziz et al. (20), Potretzke et al. (21) and Lee et al. (22) were retrospective studies. Lee et al. (22) compared both techniques using surgical approach instead of percutaneous approach.
In the current study, we included patients with HCCs up to three lesions with no lesion more than 5 cm in diameter. There were no statistically significant differences between both groups regarding number and sizes of lesions. Shibata et al. (14), Xu et al. (23) and Violi et al. (15) included subjects with lesions up to 4 cm while Ohmoto et al. (17) included subjects with lesions up to 2 cm. Potretzke et al. (21) included lesions up to 4.5 cm. Similar to our study, Ding et al. (18), Zhang et al. (19) and Abdelaziz et al. (20) included subjects with HCCs up to 5 cm. On the other hand, Lee et al. (22) included lesions up to 6 cm while the largest lesion that were included by Lu et al. (16) was 7.2 cm.
In our study, a follow up triphasic CT was done 4 weeks after the procedure to ensure complete ablation. If there was residual activity another session was done and follow up triphasic CT was performed after another 4 weeks. Thirty lesions from a total 34 lesions (88.2%) in the RFA group were completely ablated after single session, while 32 lesions from a total 34 lesions (94.1%) in the MWA were completely ablated after single session, but this was not statistically significant difference (P=0.673). These results are similar to that were reported by Lu et al. (16), Qian et al. (24), Zhang et al. (19), Ding et al. (18), Abdelaziz et al. (20), Vogl et al. (25), Lee et al. (22) and Violi et al. (15) who reported no statistically significant differences between RFA and MWA regarding achievement of complete tumor ablation, ranging from 83.4% to 98.5%. On the other hand, the number of sessions per nodule was significantly smaller in the RFA group than MWA group in Shibata et al. (14) study, but this study was performed on 1999 and 2000 using the old version of microwave apparatus. On contrary, Xu et al. (23) reported significantly higher complete ablation in the MWA group.
In current study, local tumor recurrence didn’t differ statistically between both groups. At the 6th month follow up, local recurrence was 0% (0/26) at the RFA group while it was 8% (2/24) at the MWA group (P=0.225). At 1-year follow up, local recurrence was 9% (2/22) in both groups. The Kaplan-Meier estimate for local tumor recurrence free survival at 1-year was 90.9% at RFA group and was 92.3% at MWA group with no statistically significant difference (P=0.932).
These results regarding local tumor recurrence were in concordance to that reported by Xu et al. (23), Lu et al. (16), Qian et al. (24), Zhang et al. (19), Vogl et al. (25), Lee et al. (22) and Violi et al. (15) who also found no statistically significant differences between both procedures. On the other hand, Abelaziz et al. (20) and Potretzke et al. (21) reported significantly lower local tumor recurrence with usage of MWA for HCC treatment. On the contrary, the study performed by Shibata et al. (14) which was done using the old generation of MWA apparatus showed significantly higher local tumor recurrence. Also, Ding et al. (18) reported higher local recurrence after MWA in their study, but they explained this by the larger sizes of lesions in the MWA group compared to the RFA group. A meta-analysis done by Facciorusso et al. (26) which included seven studies didn’t find any significant difference between both techniques regarding local tumor recurrence rates.
There was no statistically significant difference regarding development of de novo HCC lesions in both groups. This was in concordance with results published by Lu et al. (16), Abdelaziz et al. (20), Lee et al. (22) and Violi et al. (15).
In our study, there were no significant differences between both groups regarding Child-Pugh score at 3, 6 and 12 months of follow up. To our knowledge, no other authors compared the two techniques regarding these parameters.
Regarding negative impacts, there were no statistically significant differences between both techniques in our study. One subject in the MWA group experienced bleeding from the tumor that needed embolization. Another patient in the MWA group experienced an attack of hematemesis from esophageal varices the day after the procedure. Regarding 30-day mortality, 2 patients died within 30 days after MWA versus no one died within the same period after RFA, but this was also statistically insignificant. This was in concordance with the results of the meta-analysis done by Facciorusso et al. (26) that showed higher rate of major complications after MWA, like hemothorax, intrahepatic hematoma and intraperitoneal hemorrhage requiring blood transfusion, but was also not statistically significant finding. This comes against the early fears of higher complications after MWA due to broader ablation zones.
The mortality rate at 1 year was 15.3% in the RFA group (4 out of 26) vs. 14.8% in the MWA group (4 out of 27). This was in concordance with previous studies which showed generally comparable survival rates after both techniques.
Limitations of our study include the small sample size but we had decided to consider all patients referred to our unit during a whole period of 6 months to be included in this study. We randomized study subjects using simple randomization. Tumor size as a covariate that can influence the recurrent rate makes stratified randomization to be theoretically more appropriate, but this was not possible in our study as subjects were enrolled one at a time and so the baseline tumor sizes were not available before assignment. Although we used the simple method of randomization, there was no statistically significant difference between both groups regarding number of subjects with tumors less than 3 cm and those with tumors that measure 3 cm or more. Another limitation is that we didn’t analyze anatomical characteristics of tumors like location and proximity to blood vessels due to small sample size. No previous clinical study analyzed this issue in particular. This should be a point for future research.
Strengths of our study include that it is a randomized controlled trial unlike most of other studies that were retrospective. Shibata et al. (14) study was a randomized controlled one but this was done using the first generation MWA generator. Qian et al. (24) was also a randomized controlled trial but it was for a short period of follow up (5.5 months). Our study is the most recent randomized controlled trial comparing. To our knowledge, we are the only study that compared the changes in Child-Pugh score over time after both techniques.
In conclusion, RFA and MWA are comparable techniques for HCC treatment. Our group couldn’t prove the superiority of MWA over RFA.
Acknowledgements
Authors acknowledge Dr. Mona H. Ashry, Assistant professor of Public Health, Alexandria University and Dr. Dalia K. El Deeb, lecturer of Public health, Alexandria University for their assistance in statistical analysis of the data. In addition, we acknowledge Ahmed Smman, MBBCh. for his technical support.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was conducted in accordance with the provisions of the Declaration of Helsinki, as revised in 2013, and Good Clinical Practice guidelines. It was approved by the Ethics Committee of Faculty of Medicine, Alexandria University (IRB No. 00007555). An informed consent was obtained from all subjects included in the study.
References
- Galle PR. Management of Liver Cancer. Dig Dis 2016;34:438-9. [Crossref] [PubMed]
- Kudo M. Recent Trends in the Management of Hepatocellular Carcinoma with Special Emphasis on Treatment with Regorafenib and Immune Checkpoint Inhibitors. Dig Dis 2016;34:714-30. [Crossref] [PubMed]
- Han K, Kim JH, Ko GY, et al. Treatment of hepatocellular carcinoma with portal venous tumor thrombosis: A comprehensive review. World J Gastroenterol 2016;22:407-16. [Crossref] [PubMed]
- Yu SJ. A concise review of updated guidelines regarding the management of hepatocellular carcinoma around the world: 2010-2016. Clin Mol Hepatol 2016;22:7-17. [Crossref] [PubMed]
- Shi Y, Zhai B. A Recent Advance in Image-Guided Locoregional Therapy for Hepatocellular Carcinoma. Gastrointest Tumors 2016;3:90-102. [Crossref] [PubMed]
- Facciorusso A, Serviddio G, Muscatiello N. Local ablative treatments for hepatocellular carcinoma: An updated review. World J Gastrointest Pharmacol Ther 2016;7:477-89. [Crossref] [PubMed]
- Best J, Schotten C, Theysohn JM, et al. Novel implications in the treatment of hepatocellular carcinoma. Ann Gastroenterol 2017;30:23-32. [PubMed]
- Poulou LS, Botsa E, Thanou I, et al. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol 2015;7:1054-63. [Crossref] [PubMed]
- Lu DSK, Raman SS, Limanond P, et al. Influence of Large Peritumoral Vessels on Outcome of Radiofrequency Ablation of Liver Tumors. J Vasc Interv Radiol 2003;14:1267-74. [Crossref] [PubMed]
- Brace CL. Radiofrequency and Microwave Ablation of the Liver, Lung, Kidney, and Bone: What Are the Differences? Curr Probl Diagn Radiol 2009;38:135-43. [Crossref] [PubMed]
- Poggi G, Tosoratti N, Montagna B, et al. Microwave ablation of hepatocellular carcinoma. World J Hepatol 2015;7:2578-89. [Crossref] [PubMed]
- Mitchell DG, Bruix J, Sherman M, et al. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology 2015;61:1056-65. [Crossref] [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [Crossref]
- Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology 2002;223:331-7. [Crossref] [PubMed]
- Vietti Violi N, Duran R, Guiu B, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol 2018;3:317-25. [Crossref]
- Lu MD, Xu HX, Xie XY, et al. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: a retrospective comparative study. J Gastroenterol 2005;40:1054-60. [Crossref] [PubMed]
- Ohmoto K, Yoshioka N, Tomiyama Y, et al. Radiofrequency ablation versus percutaneous microwave coagulation therapy for small hepatocellular carcinomas: a retrospective comparative study. Hepatogastroenterology 2007;54:985-9. [PubMed]
- Ding J, Jing X, Liu J, et al. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol 2013;82:1379-84. [Crossref] [PubMed]
- Zhang L, Wang N, Shen Q, et al. Therapeutic efficacy of percutaneous radiofrequency ablation versus microwave ablation for hepatocellular carcinoma. PLoS One 2013;8:e76119. [Crossref] [PubMed]
- Abdelaziz A, Elbaz T, Shousha HI, et al. Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Surg Endosc 2014;28:3429-34. [Crossref] [PubMed]
- Potretzke TA, Ziemlewicz TJ, Hinshaw JL, et al. Microwave versus radiofrequency ablation treatment for hepatocellular carcinoma: a comparison of efficacy at a single center. J Vasc Interv Radiol 2016;27:631-8. [Crossref] [PubMed]
- Lee KF, Wong J, Hui JW, et al. Long-term outcomes of microwave versus radiofrequency ablation for hepatocellular carcinoma by surgical approach: A retrospective comparative study. Asian J Surg 2017;40:301-8. [Crossref] [PubMed]
- Xu HX, Xie XY, Lu MD, et al. Ultrasound-guided percutaneous thermal ablation of hepatocellular carcinoma using microwave and radiofrequency ablation. Clin Radiol 2004;59:53-61. [Crossref] [PubMed]
- Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies. Eur Radiol 2012;22:1983-90. [Crossref] [PubMed]
- Vogl TJ, Farshid P, Naguib NN, et al. Ablation therapy of hepatocellular carcinoma: a comparative study between radiofrequency and microwave ablation. Abdom Imaging 2015;40:1829-37. [Crossref] [PubMed]
- Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int J Hyperthermia 2016;32:339-44. [Crossref] [PubMed]