
Report of three cases of gastric choriocarcinomas—an emphasis on morphologic changes in the non-affected gastric mucosa
Introduction
Choriocarcinoma arises from trophoblastic cells of the uterus following normal or abnormal gestation, and rarely originates in the gonads, mediastinum, retroperitoneum or the gastrointestinal tract. Primary gastric choriocarcinoma (PGC) is very rare and was first reported in 1905 (1). Umigami et al. (2) had described a prevalence of 0.08% of primary gastric malignancies. This is a very aggressive neoplasm, which is in advanced stages at the diagnosis, with only a few months of survival, and is usually unresponsive to chemotherapy (3). This neoplasm is more common in men, with up to a 2.5:1 proportion (4,5). The main symptoms of PGC at presentation are anemia, epigastric pain and weight loss. For women (especially young), the diagnosis is under-suspected because these symptoms mimic pregnancy and gestational related diseases (6). Most PGC cases are mixed with an adenocarcinoma component and, rarely, with endodermal sinus tumor or neuroendocrine neoplasms (7-10). However, data regarding other histologic characteristics in the gastric mucosa are not well-reported. Additionally, most published cases are from Asia, with only one case published in a Latin-American woman (11). We report a case series of three Mexican patients with PCG, in line with the CARE statement (12).
Case presentations (Table 1)

Full table
Case 1
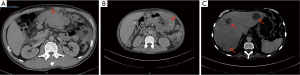
A 47-year-old man presented with a history of asthenia, adynamia, early satiety and weight loss of 16 kg in the previous eight months. Upon his physical examination, a palpable mass of about 8.0 cm with pain was discovered in his stomach. Laboratory tests revealed hemoglobin of 6.5 g/dL. Panendoscopy showed an infiltrative ulcerated tumor that extended from the body to the antral region. Tomography was performed, revealing a gastric lesion with multiple perigastric lymphadenopathies, hepatic metastasis, peritoneal carcinomatosis, and omental cake (Figure 1A,B). Another osteoblastic lesion was reported in the left sacroiliac articulation. Greatest diameter of the tumor was 9.5 cm. The biopsied specimen showed a gastric carcinoma (intestinal type) with a choriocarcinoma component. The treatment was palliative chemotherapy with cisplatin and 5-fluorouracil, due to unresectability. The patient received only one cycle and died at home one month after admission.
Case 2
A 55-year-old woman arrived with symptoms of hyporexia, asthenia, adynamia, early satiety, diffuse epigastric pain and weight loss of 15 kg. A palpable, not mobile, painful mass of 8 cm × 7 cm was noticed at physical exploration. The hemoglobin level was of 3.5 g/dL. At endoscopy, a Borrmann III tumor at the gastric body was identified. Tomography revealed ascites and metastasis to the liver. The biopsy reported a gastric adenocarcinoma with a choriocarcinoma component. The patient received palliative treatment but died six months after admission.
Case 3
A 53-year-old man was referred to this hospital with symptoms of epigastric pain, early satiety, hyporexia asthenia, adynamia and weight loss of 7 kg in one month. The laboratory test results showed hemoglobin of 4.5 g/dL and serum levels of human chorionic gonadotropin (hCG) of 4,694 mIU/mL. No mass was palpable. The endoscopy revealed a Borrmann III gastric tumor at the great curvature extending from the fundus to the antrum, with necrotic and hemorrhagic appearance. TAC showed a nodal conglomerate toward the hepatic hilum that extended to the pancreatic head (Figure 1C). The size of the lesion was 73 mm × 53 mm with an image suggestive of necrosis. The gastric mucosa was thickened. There were enlarged lymph nodes adjacent to the gastric antrum. The biopsy reported a pure gastric choriocarcinoma. The patient was treated with seven cycles of chemotherapy with Etoposide and Cisplatin (135/29 mg) with good response. The last report of hCG was 132.8 mUI/mL. The patient has survived for eight months and is still under follow-up.
Histologic findings
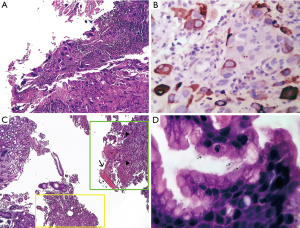
In all three cases, microscopic examination revealed a malignant tumor with extensive necrosis and recent hemorrhage, composed of large, bi- or multinucleated polygonal cells with pronounced pleomorphism and an anaplastic aspect with a broad eosinophilic cytoplasm corresponding to syncytiotrophoblast cells (Figure 2A) and large polygonal cells, with round nuclei, with prominent nucleoli and broad eosinophilic cytoplasm, corresponding to cytotrophoblast cells. The neoplastic cells were positive by immunohistochemistry for hCG, especially in the syncytiotrophoblast cells (Figure 2B). In cases 1 and 2, an adenocarcinoma component of conventional type was identified (Figure 2C), while in case 3, only a choriocarcinoma component was identified. In the adjacent gastric mucosa, follicular chronic inflammation was identified in all three cases, as well as incomplete intestinal metaplasia. In case 2 and 3, active gastritis associated with Helicobacter pylori (H. pylori) was identified (Figure 2D). In all three cases, immunohistochemistry was performed against human epidermal growth factor receptor 2 (HER2) (clone 4B5; Ventana Medical Systems Inc., Tucson, AZ), which was negative.
Discussion
We presented three cases of PGC, one pure and two as a component of a conventional adenocarcinoma and describing the characteristics of non-affected gastric mucosa. This is a very rare tumor with less than 200 cases reported in the literature, and the characteristics of the non-tumoral gastric mucosa are not described; also, only one case has been reported in Latin-American people (11).
The origin of gastric choriocarcinoma is not well-elucidated. The most plausible explanation was proposed by Pick in 1926, based on the fact that most PGC were in coexistence with an adenocarcinoma he proposed that the trophoblastic elements developed from dedifferentiation of a poorly differentiated adenocarcinoma. A pure choriocarcinoma would then arise by overgrowth of choriocarcinoma and elimination of the adenocarcinoma (13). In our cases, there was an adenocarcinoma component in two of the three cases. In the literature, the proportion of cases associated with an adenocarcinoma range from 28% to 70% (5,7,14). However, there is no series or report describing the characteristics of the surrounding gastric mucosa. We found, in all cases, follicular chronic inflammation and incomplete intestinal metaplasia. These findings are known risk factors for developing intestinal-type adenocarcinoma, which could support the hypothesis of the PGC arise from the de-differentiation or overgrowth of an intestinal-type adenocarcinoma. Finally, in two cases, we identified H. pylori.
Only the report of Fukuda et al. (10) performed HER2 in the tumor and it was negative. In our series, all tumors were HER2 negative.
Most reported cases of PGC come from Asia, with some reports in the U.S. and Europe; however, in Latin America, only few cases had been reported (11). The age of presentation ranges from 28 to 86 years (5) with a median age around 54–64 years (4,14). In our series, the median is according to these data. The survival of PGC ranges from a few days or months, with exceptional cases with long survival (15). Our cases also had poor prognosis.
In conclusion, the PGC is a rare tumor with worrisome prognosis. The clinicopathological features are comparable between populations.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Compliance with ethical standards, this study was conducted following the statements of Helsinki declaration. Informed consent of the patients or relatives was obtained.
References
- Davidsohn C. Chorioepithelium und Magekrebs, eine seltene Verschmelzung zweier bösartiger Geschwulste. Charite Ann 1905;29:426-37.
- Unakami M, Hirota E, Itabashi M, et al. 3 cases of malignant choriocarcinoma originating in the stomach. Gan No Rinsho 1982;28:204-10. [PubMed]
- Logothetis CJ, Samuels ML, Selig DE, et al. Chemotherapy of extragonadal germ cell tumors. J Clin Oncol 1985;3:316-25. [Crossref] [PubMed]
- Nam SH, Im SA, Bae KS, et al. A Case of Primary Gastric Choriocarcinoma Presenting with Amenorrhea. Cancer Res Treat 2002;34:457-60. [Crossref] [PubMed]
- Imai Y, Kawabe T, Takahashi M, et al. A case of primary gastric choriocarcinoma and a review of the Japanese literature. J Gastroenterol 1994;29:642-6. [Crossref] [PubMed]
- Larish A, Kumar A, Kerr S, et al. Primary Gastric Choriocarcinoma Presenting as a Pregnancy of Unknown Location. Obstet Gynecol 2017;129:281-4. [Crossref] [PubMed]
- Garcia RL, Ghali VS. Gastric choriocarcinoma and yolk sac tumor in a man: observations about its possible origin. Hum Pathol 1985;16:955-8. [Crossref] [PubMed]
- Hirano Y, Hara T, Nozawa H, et al. Combined choriocarcinoma, neuroendocrine cell carcinoma and tubular adenocarcinoma in the stomach. World J Gastroenterol 2008;14:3269-72. [Crossref] [PubMed]
- Satake N, Chikakiyo M, Yagi T, et al. Gastric cancer with choriocarcinoma and yolk sac tumor components: case report. Pathol Int 2011;61:156-60. [Crossref] [PubMed]
- Fukuda S, Fujiwara Y, Wakasa T, et al. Collision tumor of choriocarcinoma and small cell carcinoma of the stomach: A case report. Int J Surg Case Rep 2017;37:216-20. [Crossref] [PubMed]
- Liu Z, Mira JL, Cruz-Caudillo JC. Primary gastric choriocarcinoma: a case report and review of the literature. Arch Pathol Lab Med 2001;125:1601-4. [PubMed]
- Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case report guideline development. J Clin Epidemiol 2014;67:46-51. [Crossref] [PubMed]
- Krulewski T, Cohen LB. Choriocarcinoma of the stomach: pathogenesis and clinical characteristics. Am J Gastroenterol 1988;83:1172-5. [PubMed]
- Kobayashi A, Hasebe T, Endo Y, et al. Primary gastric choriocarcinoma: two case reports and a pooled analysis of 53 cases. Gastric Cancer 2005;8:178-85. [Crossref] [PubMed]
- Takahashi K, Tsukamoto S, Saito K, et al. Complete response to multidisciplinary therapy in a patient with primary gastric choriocarcinoma. World J Gastroenterol 2013;19:5187-94. [Crossref] [PubMed]


