
Assessment of the external validity of the AJCC 8th staging system for small intestinal adenocarcinoma: a time to reconsider the role of tumor location?
Introduction
Small intestinal adenocarcinoma represents a rare and an understudied entity within gastrointestinal malignancies (1). Staging and treatment paradigms for this disease were developed primarily following those for colon cancer (2).
Recently, the American Joint Committee on Cancer (AJCC) has updated its staging system for small intestinal adenocarcinoma in order to make it more in line with colon cancer staging (3). The most important changes put forward in the new staging system included the definition of N1 stage as metastasis in 1–2 lymph nodes and the definition of N2 stage as metastasis in three or more lymph nodes. Other subtle changes were proposed in the differentiation between T3 and T4 sub-stages (the description of the extent of penetration into retroperitoneum was omitted). Table 1 provides a summary of the three most recent versions of the AJCC staging system for small intestinal adenocarcinoma.
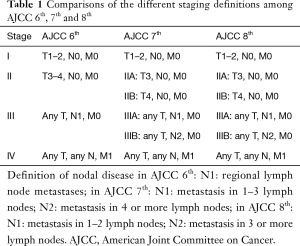
Full table
The stage is an important determinant of treatment strategies for small intestinal adenocarcinoma (in addition to other patient- and disease-related parameters) (4). Unfortunately, few studies have discussed in depth the relevance and external performance of different AJCC staging systems for small intestinal adenocarcinoma which might have left practicing physicians bewildered as to the best prognostic model to adopt when treating those patients.
It is really important to externally assess novel staging models in a population-based setting. This should confirm the importance of the changes put forward in the new system as well as clarify any possible defects to be dealt with in future staging system editions (5). Thus, the objective of the current study is to evaluate the 8th AJCC staging system for small intestinal adenocarcinomas in comparison to previous staging systems (6th and 7th systems) in a cohort of small intestinal adenocarcinoma patients derived from the surveillance, epidemiology and end results (SEER) database (6).
Methods
The current analysis was based on a study cohort extracted from the SEER-18 registry (through SEER*stat software). In order to choose the study cohort, the following criteria were considered: Diagnosis from 2004–2015 (in order to ensure completeness of staging data), ICD-O-3/WHO 2008 category of “small intestine” and histology of adenocarcinoma. Records with inadequate information about 6th AJCC stage or records with neuroendocrine histologies as well as other non-epithelial histologies were excluded. Additionally, records with inadequate information about the number of examined or dissected lymph nodes were excluded.
Relevant data were then sought from each record in the included cohort; including: age at diagnosis, gender, ethnicity, sub-site within the intestine, stage (according to AJCC 6th, 7th, and 8th systems), grade, diagnosis confirmation, staging approach (clinical or pathological), surgical treatment, sites of distant metastases (for patients diagnosed starting from 2010). Survival parameters were also collected (including vital status, survival months and cause of death). Cancer-specific survival was defined as the time from small intestinal adenocarcinoma diagnosis to death from small intestinal adenocarcinoma.
Statistical analyses
For overall survival analyses according to AJCC 6th, 7th, and 8th systems, Kaplan-Meier survival calculation together with log/rank testing were used. Cancer-specific Cox regression hazard analyses were also evaluated for the three systems (adjusted for age, race, gender, sub-site, grade and surgical treatment). Concordance index (c-statistic) was also calculated for the three staging systems. Death from small intestinal adenocarcinoma was used as the dependent variable (7). All reported P values were two-sided. All analyses were performed through SPSS program (v.20, NY, IBM).
Results
A total of 2,997 patients with small intestinal adenocarcinoma (diagnosed 2004–2015) were included in the study cohort. The age group of 40–69 years represented 56.7% and males comprised 54.9% of the study cohort. Table 2 clarifies other baseline characteristics of the study cohort. Distribution of patients according to the three staging systems was also revealed. Clinically-staged patients represented 3.6% of the study cohort. Ninety-four percent of the patients were treated with some form of radical surgery. Fifty-nine point five percent of the patients have lymph node dissection of at least 12 lymph nodes. Because chemotherapy and radiotherapy were reported generally as a yes or no/unknown options without reporting of their details (i.e., dose/schedule) and the certainty of reporting is not confirmed (i.e., some patients with no/unknown category could have received chemotherapy or radiotherapy without corresponding reporting in the database), they were not included into further survival analysis.
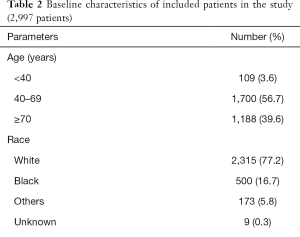
Full table
Survival outcomes
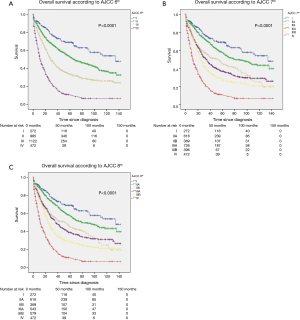
Overall survival was compared according to the three AJCC staging systems. For the three versions, the P value for the trend in overall survival was significant (P<0.0001) (Figure 1A,B,C).
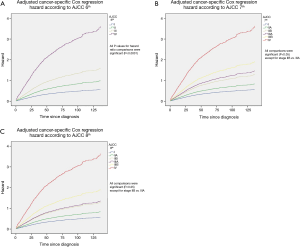
The cancer-specific Cox regression hazard (adjusted for age, race, gender, sub-site, grade and surgical treatment) was calculated for the three staging systems. Pairwise hazard ratio comparisons between different AJCC 6th stages were conducted and all P values for comparisons were significant (P<0.0001) (Figure 2A). Pairwise hazard ratio comparisons between different AJCC 7th and 8th stages were also performed and all comparisons were significant (P<0.05) except for stage IIB vs. IIIA (Figure 2B,C).
C-statistic (using death from small intestinal adenocarcinoma as the dependent variable) for AJCC 6th staging system was: 0.645 [standard error (SE): 0.011; 95% CI: 0.623–0.668]; for c-statistic for AJCC 7th staging system was 0.658 (SE: 0.011; 95% CI: 0.637–0.680); while c-statistic for AJCC 8th staging system was 0.660 (SE: 0.011; 95% CI: 0.638–0.682).
When the c-statistic calculation was repeated in the subset of patients with adequate lymph node dissection (at least 12 lymph nodes), similar c-statistic results were obtained for the three staging system.
Prognostic value of lymph node ratio and primary tumor localization
An additional assessment of the prognostic value of lymph node ratio was conducted through a Cox regression model for cancer-specific survival adjusted for age, race, gender, sub-site, grade and surgical treatment. In that model, higher lymph node ratio was strongly predictive of worse cancer-specific survival (P<0.0001). Notably within the same survival model, other parameters associated with worse cancer-specific survival include older age (≥70 years old) (P=0.005) and duodenal localization of the primary (P=0.008).
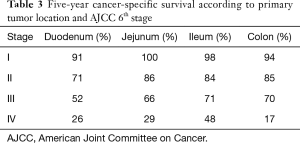
In order to elaborate more on the impact of primary tumor localization on the outcomes of small intestinal adenocarcinoma, an additional assessment of the 5-year cancer-specific survival was conducted according to AJCC 6th stage and primary tumor location. Consistent with the above findings, the duodenal primary has the worst 5-year cancer-specific survival across all stages (Table 3). An additional comparison was done to the 5-year cancer-specific survival of a SEER-based colon cancer cohort (203,810 patients) diagnosed during a similar period [2004–2014] according to AJCC 6th stage (Table 3). Overall, colon cancer survival seems to be similar to that of the jejunum and ileum and better than that of the duodenum.
Full table
Discussion
The objective of the current analysis is to evaluate the external validity of the new AJCC 8th staging system for small intestinal adenocarcinoma compared to previous AJCC systems. Unfortunately, there is no evidence that AJCC 8th system provided better prognostic characterization compared to previous staging systems. Lymph node ratio might play a better role in the prognostic stratification of node-positive small intestinal adenocarcinoma. Moreover, incorporation of sub-site-specific staging paradigms might be needed in the future for different small intestinal sub-sites.
The primary strong point of the current analysis is the incorporation of a large number of patients (relative to the prevalence of this uncommon disease). On the other hand, the weaknesses of the current analysis are typical of those encountered with other population-based studies. These include the lack of some therapeutic details for each patient (particularly chemotherapy and radiotherapy). Similarly, SEER datasets do not provide information about co-morbidities or the performance of included patients. It has to be noted that whereas the absence of information about co-morbidities might affect overall survival estimates; it should not affect cancer-specific survival estimates.
While the proposed sub-classifications of node-positive (stage III) patients do not seem to improve prognostic assessment in the AJCC 7th and 8th staging systems, lymph node ratio appears a more plausible method for risk-stratifying those patients. This finding is in line with multiple similarly conducted studies in other solid tumors and this parameter needs to be considered in future versions of the staging system (8). This finding is also in line with previously published population-based studies in small intestinal adenocarcinoma (9).
The current analysis additionally suggested that duodenal localization of the primary tumor is associated with worse survival outcomes. This is in line with previously published population-based studies (10). Possible reasons for the difference in outcomes for duodenal versus other small intestinal sub-sites might be related to genetic differences between these sub-sites (adenocarcinoma of the distal parts of the small intestine might behave more like colon adenocarcinoma; while adenocarcinoma of the proximal parts might behave more like upper gastrointestinal adenocarcinoma) (11-13). This is also demonstrated in the current analysis by the relevant similarity in outcomes between colon cancer and jejunal/ileal adenocarcinoma versus duodenal adenocarcinoma. Another reason for differences between different subsites of small intestinal adenocarcinoma might relate to the relative difficulty to obtain a margin-negative radical surgical resection for duodenal primary versus other small intestinal primaries (14). This is an important area for future research because a “one size fits all” strategy does not seem to apply to all small intestinal adenocarcinomas.
The changes put forward in the AJCC 8th staging system for small intestinal adenocarcinoma seem to try to mirror colon cancer staging system (particularly with regards to the N staging) (15). However, and as clarified above, it seems from the current analysis that this is not the best way to approach small intestinal adenocarcinoma staging and tailored staging approach need to be pursued for this disease. Particular attention might be paid to developing different staging systems for duodenum versus jejunum/ileum because of demonstrated differences in prognosis.
The three AJCC editions deal with metastatic disease (M1) as a single group. However, it has to be noted that previous population-based studies in a number of malignancies proposed that the outcome of patients with metastatic disease may vary based on the number and site(s) of distant metastases (16-19). Because the distribution of distant metastases was available only for small intestinal adenocarcinoma patients diagnosed from 2010, it was not feasible to assess this scenario in the current dataset. Further studies are needed to evaluate this point.
Although the c-statistic for the three staging systems was above 0.5, all of them were lower than 0.7. This indicates that the three versions of the AJCC staging system have limited ability in terms of predicting cancer-specific survival, and there is still a significant space for improving staging systems for small intestinal adenocarcinomas.
The 8th edition of the AJCC staging system for many solid tumors was enriched by more integration of molecular markers into the stage grouping systems (e.g., breast, prostate and oropharyngeal cancer staging systems) (20,21). It is still to be seen if integration of relevant markers for small intestinal adenocarcinoma (e.g., RAS or BRAF or microsatellite instability) might play a role in the refinement of the staging system for small intestinal carcinoma.
In conclusion, there is no evidence that AJCC 8th system provided better prognostic characterization compared to previous AJCC staging systems for small intestinal adenocarcinoma. Further research is needed to explore better prognostic models for those patients (e.g., models incorporating tumor location or lymph node ratio).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Aparicio T, Zaanan A, Mary F, et al. Small Bowel Adenocarcinoma. Gastroenterol Clin North Am 2016;45:447-57. [Crossref] [PubMed]
- Aparicio T, Zaanan A, Svrcek M, et al. Small bowel adenocarcinoma: epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis 2014;46:97-104. [Crossref] [PubMed]
- Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93-9.
- Jun SY, Kim M, Jin Gu M, et al. Clinicopathologic and prognostic associations of KRAS and BRAF mutations in small intestinal adenocarcinoma. Mod Pathol 2016;29:402-15. [Crossref] [PubMed]
- Siontis GC, Tzoulaki I, Castaldi PJ, et al. External validation of new risk prediction models is infrequent and reveals worse prognostic discrimination. J Clin Epidemiol 2015;68:25-34. [Crossref] [PubMed]
- Surveillance, Epidemiology and End Results Program. About the SEER Program. Accessed June 25, 2016. Available online: http://seer.cancer.gov/about
- Ranstam J. Multiple P-values and Bonferroni correction. Osteoarthritis Cartilage 2016;24:763-4. [Crossref] [PubMed]
- Dolan RD, McSorley ST, Horgan PG, et al. Determinants of lymph node count and positivity in patients undergoing surgery for colon cancer. Medicine (Baltimore) 2018;97:e0185. [Crossref] [PubMed]
- Tran TB, Qadan M, Dua MM, et al. Prognostic relevance of lymph node ratio and total lymph node count for small bowel adenocarcinoma. Surgery 2015;158:486-93. [Crossref] [PubMed]
- Wilhelm A, Galata C, Beutner U, et al. Duodenal localization is a negative predictor of survival after small bowel adenocarcinoma resection: A population-based, propensity score-matched analysis. J Surg Oncol 2018;117:397-408. [Crossref] [PubMed]
- Shenoy S. Genetic risks and familial associations of small bowel carcinoma. World J Gastrointest Oncol 2016;8:509-19. [Crossref] [PubMed]
- Hänninen UA, Katainen R, Tanskanen T, et al. Exome-wide somatic mutation characterization of small bowel adenocarcinoma. PLoS Genet 2018;14:e1007200. [Crossref] [PubMed]
- Schrock AB, Devoe CE, McWilliams R, et al. Genomic Profiling of Small-Bowel Adenocarcinoma. JAMA Oncol 2017;3:1546-53. [Crossref] [PubMed]
- Vagholkar K, Mathew T. Adenocarcinoma of the small bowel: a surgical dilemma. Saudi J Gastroenterol 2009;15:264-7. [Crossref] [PubMed]
- Abdel-Rahman O. Revisiting Dukes' paradigm; some node positive colon cancer patients have better prognosis than some node negative patients. Clin Transl Oncol 2018;20:794-800. [Crossref] [PubMed]
- Abdel-Rahman O. Clinical correlates and prognostic value of different metastatic sites in metastatic renal cell carcinoma. Future Oncol. 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Abdel-Rahman O. Clinical correlates and prognostic value of different metastatic sites in patients with malignant melanoma of the skin: a SEER database analysis. J Dermatolog Treat 2018;29:176-81. [Crossref] [PubMed]
- Oweira H, Petrausch U, Helbling D, et al. Prognostic value of site-specific extra-hepatic disease in hepatocellular carcinoma: a SEER database analysis. Expert Rev Gastroenterol Hepatol 2017;11:695-701. [Crossref] [PubMed]
- Oweira H, Petrausch U, Helbling D, et al. Prognostic value of site-specific metastases in pancreatic adenocarcinoma: A Surveillance Epidemiology and End Results database analysis. World J Gastroenterol 2017;23:1872-80. [Crossref] [PubMed]
- Abdel-Rahman O. Validation of American Joint Committee on Cancer eighth staging system among prostate cancer patients treated with radical prostatectomy. Ther Adv Urol 2017;10:35-42. [Crossref] [PubMed]
- Abdel-Rahman O. Validation of the 8th AJCC prognostic staging system for breast cancer in a population-based setting. Breast Cancer Res Treat 2018;168:269-75. [Crossref] [PubMed]


