
Long-term follow-up after conventional transarterial chemoembolization (c-TACE) with mitomycin for hepatocellular carcinoma (HCC)
Introduction
Transarterial chemoembolization (TACE) is commonly used in patients with unresectable hepatocellular carcinoma (HCC) and is believed to be implemented in almost 50% of all patients at some point during their treatment course (1). The rationale supporting this approach is the increased overall survival (OS) achieved with TACE compared to conservative management as shown by two meta-analyses of randomized controlled trials (RCTs) (2,3).
In these two meta-analyses, multiple techniques were applied including different embolic agents and chemotherapeutic drugs, with or without ethiodized oil. This agent functions as a carrier for the chemotherapeutic drug, as well as an embolic agent that can travel distally into the tumor vasculature (4).
Recently, new strategies not involving an oil-based agent have been applied for intra-arterial treatment of HCC, including drug-eluting beads (DEB-TACE) and radioactive beads (Y-90 radioembolization). Despite some potential advantages there is still no evidence demonstrating survival superiority of one regime over another (5,6).
Conventional TACE (c-TACE), which includes utilization of lipiodol, remains a safe and effective approach to treat HCC as demonstrated by a large systematic review including over 10,000 patients (7). In this review, OS was 70.3% at 1 year, 51.8% at 2 years, 40.4% at 3 years, and 32.4% at 5 years. The most common adverse events included liver enzyme abnormalities and symptoms associated with post embolization syndrome.
The purpose of this study is to perform a retrospective analysis of c-TACE with Mitomycin as the single chemotherapeutic agent in patients with HCC and to determine safety and efficacy of this method, as well as potential prognostic factors.
Methods
After Institution Review Board (IRB: Pro00061253) approval, retrospective analysis was conducted from 2007 to 2012 including all patients with HCC submitted to c-TACE with Mitomycin as the only loco-regional therapy. Informed consent from participants was waived by the IRB. Inclusion criteria were patients ≥18 years old, ECOG 0-2, diagnosed with HCC according to prior guidelines (8) and Barcelona Clinic Liver Cancer (BCLC) stages A–C. Patients who were submitted to other types of loco-regional therapy (i.e., percutaneous ablation, TACE with drug eluting beads, radioembolization) were excluded.
Data collection included age, sex, diabetes, hypertension, coronary artery disease, smoking history, cause of cirrhosis, Child-Pugh classification, Model for End-Stage Liver Disease (MELD) score, and number of c-TACE procedures. Laboratory data included alpha-fetoprotein (AFP), albumin, bilirubin and complete blood cell count, in addition to platelet-to-lymphocyte (P/L) ratio, neutrophil-to-lymphocyte (N/L) ratio, and albumin-to-bilirubin (A/B) ratio.
Imaging data included number and size of lesions, portal vein invasion and metastatic lesions. In addition, degree of ethiodized oil uptake by the lesions was assessed by CT within the first month post procedure and categorized as <50%, 50–80% and >80% of lesion coverage.
Efficacy was determined by OS at 1, 3 and 5 years, censored by date of death or last follow-up visit. Treatment response was assessed according to mRECIST criteria on cross-sectional imaging at 1-month follow-up after the last c-TACE procedure. Adverse events were recorded according to the Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Plausible prognostic factors were analyzed by multiple linear regression analysis. Significance levels were set at 0.05 for all tests.
Conventional TACE
c-TACE was performed through femoral access under moderate sedation provided with midazolam and fentanyl. Sub-segmental or segmental treatments were performed whenever possible. If not, lobar infusion was achieved. All embolizations were performed through a 2.8-Fr micro-catheter (Progret® Terumo, Somerset, NJ, USA). A 10 mL solution containing an empiric dose of 10 mg of mitomycin was combined with 5 mL of ethiodized oil (Lipiodol® Guerbet, Villepinte, France), 10 mL of iodine contrast media (Visipaque® GE Healthcare, Waukesha, WI, USA) and 10 mL of normal saline. This solution was distributed in two 30 mL syringes and mixed via a 3-way stopcock until a homogenous solution was obtained (10–15 cycles). The total volume was equally divided in two different containers and one vial of polyvinyl alcohol (PVA) particles 300–500 µm (Cook, Bloomington, IN, USA) was added to one of them. The mixture without PVA was infused first until saturation of tumoral vasculature with ethiodized oil was observed. This was followed by the infusion of the solution containing the embolic particles (PVA). The endpoint was no further tumor vascular enhancement and complete flow stasis within the feeding vessels. Patients were admitted for overnight observation. The number of c-TACE sessions was determined at the discretion of the interventionalist and performed 6–8 weeks apart.
Results
A total of 60 patients were identified and characteristics are listed in Table 1. OS rate at 1, 3 and 5 years was 72.1%, 47.8% and 39.3%, respectively. Median OS was 15 months (range, <1–60.9 months). When stratified by disease stage the 1-, 3-, 5-year OS rate was 76%, 53.3% and 42.4% for very early/early, 75.2%, 47.3% and 40.5% for intermediate and 50.5%, 32.1% and 32.1% for advanced stage, respectively, without statistical significance (Table 2). Sixteen patients had liver transplantation and 11 were alive by the time of analysis.

Full table

Full table
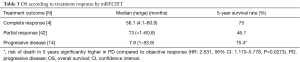
Tumor response by mRECIST criteria was complete (CR) in 4 patients (6.7%), partial (PR) in 42 patients (70.0%) and progression (PD) in 14 patients (23.3%) (Table 3). Objective response, defined as combination of CR and PR patients, was 76%. When stratified by tumor response, median OS in the CR group was 56.1 months (range, 4.1–60.9 months) with a 75% chance of survival in 5 years. In the PR group, median OS was 13 months (range, <1–60.8 months) with a 46.1% chance of survival is 5 years. PD group had median OS of 7.8 months (range, 1–53.9 months) with a 15.4% chance of survival in 5 years (Table 3). Risk of death in patients with PD in 5 years was significantly higher compared to patients with objective response (HR: 2.531, 95% CI: 1.110–5.778, P=0.0273).
Full table
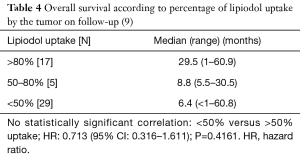
Lipiodol uptake analysis was available in 51 patients that had a CT within the first month of the initial procedure. Less than 50% uptake was observed in 29 patients (57%), between 50–80% uptake was seen in 5 patients (10%) and more than 80% uptake was observed in 17 patients (33%). Median (range) OS in months was 6.4 (<1–60.8), 8.8 (5.5–30.5) and 29.5 (1–60.9) for uptake of <50%, between 50–80% and >80%, respectively. There was no statistically significant difference in OS in patients with higher lipiodol uptake compared to less uptake (<50% versus >50% uptake; HR: 0.713, 95% CI: 0.316–1.611; P=0.4161) (Table 4). Complications were limited to one grade 1 (tachycardia; 1.6%) and one grade 3 (ischemic cholecystitis, requiring surgical removal; 1.6%).
Full table
Discussion
Intra-arterial therapy is one of the pillars in the management of HCC (1). Many methods are available including bland embolization, c-TACE, DEB-TACE and radioembolization (5,6). c-TACE has long been proven an effective strategy to treat HCC (10), with improved OS compared to conservative management (26% versus 3% 3-year survival, P=0.002). Similarly, this single institutional retrospective study demonstrated that the c-TACE was safe and effective.
The OS rates at 1, 3 and 5 years were 72.1%, 47.8% and 39.3%, respectively. Median OS was 15 months (range, <1–60.9). This is comparable to the recent systematic review including over 10,000 patients submitted to c-TACE where 1-, 3- and 5-year survival rates were 70.3%, 40.4% and 32.4%, respectively, and median OS was 19.4 months (7). In this same review, the overall mortality rate was 0.6% and most deaths were related to hepatic failure (7). In this study, there was no death related to the procedure and only 1 (1.7%) major complication occurred (ischemic cholecystitis).
Radiological response is a predictor of survival after local-regional therapy for hepatic tumors as shown by Memon et al. (11). Patients who responded to the treatment based on the European Association for the Study of the Liver (EASL) criteria had longer survival than non-responders (P=0.0001) (11,12). In the present study, similar findings were observed since patients with disease progression by mRECIST criteria had significantly higher risk of death in 5 years compared to patients with objective response (HR: 2.531, 95% CI: 1.110–5.778, P=0.0273).
Greater lipiodol uptake has been shown to be associated with better outcome, as described by prior publication including 490 patients that demonstrated significantly longer OS in patients with compact lipiodol uptake compared to patients with non-compact uptake (1-, 3- and 5-year survival rates of 92.7%, 70.7% and 52.4% versus 60.8%, 28.0% and 16.9%; P=0.001) (12). In this present study although not statistically significant there was a trend to increased OS in patients with higher lipiodol uptake (29.5, 8.8 and 6.4 months with >80%, 50–80%, <50% uptake, respectively). Lipiodol accumulation within the tumor has been shown to be a predictor of tumor necrosis as demonstrated by Takayasu et al., who compared CT findings with resected specimens. In a total of 41 tumors good correlation (r=83) was found between retained lipiodol and necrotic tissue under pathological analysis Table 4 (9). Mitomycin is a well know anti-tumor agent, mostly used for the treatment of gastric and pancreatic adenocarcinoma. It acts by cross-linking and alkylation of DNA (13). Many studies have shown its applicability to treat HCC in the setting of TACE either as single chemotherapeutic agent or in combination with others (14-16).
As mentioned before, until now there is no evidence to support the superiority of one specific type of intra-arterial therapy over another in terms of OS, including single chemotherapeutic agent versus multiple drugs. A recent RCT showed 1- and 2-year survival rates of 86.2% and 56.8% after DEB-TACE and 83.5% and 55.4% after c-TACE with lipiodol (P=0.949) (17). Another RCT despite showing better objective response (52% versus 44%) and lower side effects (P=0.0001) with DEB-TACE failed to meet superiority over c-TACE with lipiodol (5). Similarly, Y-90 embolization was shown to have significantly improved time to progression (TTP) compared to c-TACE with lipiodol (>26 versus 6.8 months, P=0.0012), but did not show OS benefit (median of 18.6 versus 17.7 months, P=0.99) (6).
The effect of lipiodol is based on its capability to function as a carrier of the chemotherapeutic agent and its prolonged retention by malignant cells, leading to higher drug concentration within the tumor (18). In addition, given its oily propriety lipiodol can travel through the microcirculation reaching the distal portal vein branches and hepatic sinusoids. This can lead to dual embolization as arterial and portal blood supply are terminated (19). Therefore, treatment response can be improved since portal vein supply plays an important role in tumoral vascularization, especially when arterial flow is suppressed (20,21).
This study was limited by its retrospective nature and relative small sample size. Also, it is worth mentioning the technical aspect of using an oil-in-water emulsion (1:6) and not the opposite. Water-in-oil emulsion was shown to have significantly higher carriage capacity and longer release time for the chemotherapeutic agent (22,23). In addition, there was no report on the exact amount of lipiodol and chemotherapeutic agent injected in each session. This can vary a lot depending on the tumor size and presence of arterio-venous shunts, and may influence treatment response. Finally, although super-selective or selective catheterization was attempted whenever possible, in many instances lobar infusion was performed. This could have affected tumor response, leading to the relative low complete response rate in this series (6%) (24).
In conclusion, c-TACE with lipiodol and Mitomycin was effective and safe in this patient population, achieving similar OS as recent publications, with minimal complications. Risk of death was significantly higher in patients without tumor response. Increased lipiodol uptake may be associated with improved OS.
Acknowledgements
This study was sponsored by Guerbet.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institution Review Board of Medical University of South Carolina (IRB: Pro00061253).
References
- Lencioni R, Kudo M, Ye SL, et al. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): second interim analysis. Int J Clin Pract 2014;68:609-17. [Crossref] [PubMed]
- Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 2002;224:47-54. [Crossref] [PubMed]
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003;37:429-42. [Crossref] [PubMed]
- Chen MS, Li JQ, Zhang YQ, et al. High-dose iodized oil transcatheter arterial chemoembolization for patients with large hepatocellular carcinoma. World J Gastroenterol 2002;8:74-8. [Crossref] [PubMed]
- Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol 2010;33:41-52. [Crossref] [PubMed]
- Salem R, Gordon AC, Mouli S, et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology 2016;151:1155-1163.e2. [Crossref] [PubMed]
- Lencioni R, de Baere T, Soulen MC, et al. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology 2016;64:106-16. [Crossref] [PubMed]
- Arslanoglu A, Seyal AR, Sodagari F, et al. Current Guidelines for the Diagnosis and Management of Hepatocellular Carcinoma: A Comparative Review. AJR Am J Roentgenol 2016;207:W88-98. [Crossref] [PubMed]
- Takayasu K, Arii S, Matsuo N, et al. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol 2000;175:699-704. [Crossref] [PubMed]
- Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 2002;35:1164-71. [Crossref] [PubMed]
- Memon K, Kulik L, Lewandowski RJ, et al. Radiographic response to locoregional therapy in hepatocellular carcinoma predicts patient survival times. Gastroenterology 2011;141:526-35. [Crossref] [PubMed]
- Kim DY, Ryu HJ, Choi JY, et al. Radiological response predicts survival following transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma. Aliment Pharmacol Ther 2012;35:1343-50. [Crossref] [PubMed]
- Tomasz M, Palom Y. The mitomycin bioreductive antitumor agents: cross-linking and alkylation of DNA as the molecular basis of their activity. Pharmacol Ther 1997;76:73-87. [Crossref] [PubMed]
- Gruber-Rouh T, Schmitt C, Naguib NNN, et al. Transarterial chemoembolization (TACE) using mitomycin and lipiodol with or without degradable starch microspheres for hepatocellular carcinoma: comparative study. BMC Cancer 2018;18:188. [Crossref] [PubMed]
- Sahara S, Kawai N, Sato M, et al. Prospective evaluation of transcatheter arterial chemoembolization (TACE) with multiple anti-cancer drugs (epirubicin, cisplatin, mitomycin c, 5-fluorouracil) compared with TACE with epirubicin for treatment of hepatocellular carcinoma. Cardiovasc Intervent Radiol 2012;35:1363-71. [Crossref] [PubMed]
- Marelli L, Stigliano R, Triantos C, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol 2007;30:6-25. [Crossref] [PubMed]
- Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer 2014;111:255-64. [Crossref] [PubMed]
- Nakakuma K, Tashiro S, Hiraoka T, et al. Studies on anticancer treatment with an oily anticancer drug injected into the ligated feeding hepatic artery for liver cancer. Cancer 1983;52:2193-200. [Crossref] [PubMed]
- Kan Z. Dynamic study of iodized oil in the liver and blood supply to hepatic tumors. An experimental investigation in several animal species. Acta Radiol Suppl 1996;408:1-25. [PubMed]
- Kan Z, Madoff DC. Liver anatomy: microcirculation of the liver. Semin Intervent Radiol 2008;25:77-85. [Crossref] [PubMed]
- de Baère T, Denys A, Briquet R, et al. Modification of arterial and portal hemodynamic after injection of iodized oil in the hepatic artery: experimental study. J Vasc Interv Radiol 1998;9:305-10. [Crossref] [PubMed]
- Kan Z, Wright K, Wallace S. Ethiodized oil emulsions in hepatic microcirculation: in vivo microscopy in animal models. Acad Radiol 1997;4:275-82. [Crossref] [PubMed]
- de Baere T, Zhang X, Aubert B, et al. Quantification of tumor uptake of iodized oils and emulsions of iodized oils: experimental study. Radiology 1996;201:731-5. [Crossref] [PubMed]
- de Baere T, Arai Y, Lencioni R, et al. Treatment of Liver Tumors with Lipiodol TACE: Technical Recommendations from Experts Opinion. Cardiovasc Intervent Radiol 2016;39:334-43. [Crossref] [PubMed]


