
Increased circulating levels of vascular endothelial growth factor C can predict outcome in resectable gastric cancer patients
Introduction
Lymphangiogenesis plays a key role in the progression and metastatization processes of several malignancies. Lymphatic spread is a frequent and early feature in gastric cancer (GC) correlated to different variables such as the depth of wall invasion, the tumor grading and size (1-4). Therefore, lymph node metastasis is one of the most important prognostic factor in GC patients (pts) and, consequently, the extension of lymphadenectomy is a relevant issue of surgical treatment so much to condition the outcome of pts who receive a potentially curative treatment (5-9). Vascular endothelial growth factor-C (VEGF-C) has proven to be pivotal for lymphangiogenesis (10-11) through the binding to its receptor VEGFR3 (Flt-4) (12) which is primarily expressed on the endothelium of lymphatic vessels (13). Ligand binding activates the downstream signaling pathway and promotes endothelial cell growth, survival and lymphangiogenesis (14). VEGF-C also promotes tumor lymphangiogenesis and lymph node metastasis in preclinical models (15-17) and increased levels of this factor are associated with a poor prognosis in different cancer types (18,19). Data from mice tumor xenografts suggest that VEGF-C from the transplanted GC cells could induce lymphangiogenesis (20) with higher tumor lymphatic vessel density and lymph node metastasis (21,22). Despite these models, few studies have tried to evaluate the prognostic value of VEGF-C serum levels in patients with resectable GC until now.
In this study we investigate the role of high levels of circulating VEGF-C in resectable GC pts in order to determine its relationship to clinicopathological parameters and clinical outcome, assuming a possible application in the clinical practice.
Methods
Patients
Patients aged ≥18 years affected by histologically confirmed gastric or gastroesophageal (GEJ) adenocarcinoma, with no radiologic evidence of metastasis at the preoperative staging and no prior anti-cancer treatment, were considered eligible for our study. The protocol was approved by the institutional board at the center and the study was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice. All pts provided a written informed consent before the enrollment in the study.
Radiological assessment was performed within 28 days before surgery [chest and abdomen computed tomography (CT) scan in all pts; bone scan, brain CT scan or magnetic resonance imaging (MRI) as needed]. Furthermore, each pts underwent full clinical history, physical examination, biochemical and blood samples before the intervention.
All pts with resectable GC underwent surgical treatment and the approach was chosen by surgeons based on the primary tumor site. R0 resection was defined as a surgery without micro- or macroscopic residual disease, as detected by anatomo-pathological analysis. R1 and R2 resections were defined as the presence of cancer cells microscopically detected at the margin or at “naked eye” respectively.
The following variables were recorded in all pts after the surgery: type of surgery, Eastern Cooperative Oncology Group performance status (ECOG criteria on a 5-point scale, with 0 indicating no symptoms and higher numbers indicating greater disability) (23), tumor site, pathological disease’s stage (24) tumor grading and histotype.
Postoperatively, pts with stage pT1 and pT2N0 underwent exclusive follow-up, whereas pts with pT3-4N0 or every pN+ received adjuvant chemotherapy based on fluoropyrimidine monotherapy schedule or in combination with oxaliplatin for 6 months. After the treatment, all pts were followed-up every 3 months for the first 2 years, every 6 months from 3rd to 5th year and every year thereafter.
Serum VEGF-C levels
Venous blood samples were collected from pts the day before surgery in order to determine the serum circulating VEGF-C level. The blood donations of healthy control subjects were used to define the normal serum values of the protein. Blood sample were centrifuged and stored at −80 °C until the analysis, which has performed within 1 week from the collection. Serum levels of the protein were assessed using commercially available sandwich kits (R&D Systems, Minneapolis, MN, USA) by enzyme-linked immunoadsorbent assay (ELISA) with a detection limit <8 pg/mL. Samples were prepared and tested in duplicate according to the manufacturers’ instructions of ELISA kits, specific for human VEGF-C and not cross-reacting with other known cytokines.
Statistical analysis
SPSS software (version 21.00; SPSS, Chicago, IL, USA) was used for statistical analysis. Data are expressed as median, range and 95% confidence interval (CI). The cutoff for high and normal serum VEGF-C level in GC pts was defined by the median preoperative value of the protein.
We considered overall survival (OS) as the time elapsed between the diagnosis and the death of the patient from any cause and disease-free survival (DFS) as the time from the diagnosis until recurrence of tumor or death from any cause. OS and DFS distributions were estimated by the Kaplan-Meier method with 95% CI. Differences in OS and DFS were evaluated by the log-rank test and described by the Kaplan-Meier method. Univariate statistical analysis was assessed by the Mantel-Cox test, using median values as cut-off to group pts in case of continuous variables. Cox proportional-hazards model was applied to multivariate survival analysis, in which all significant variables in the univariate model were used. Values P<0.05 indicated statistical significance. Furthermore, differences between groups of variables were assessed by non-parametric tests: Mann-Whitney U, Kruskal-Wallis and multiple comparisons analysis were used for median comparisons for two and more than two groups, respectively. Pearson rank test was used to evaluate correlations.
Results
From January 2004 until December 2009, 194 pts were enrolled in the study. Of these pts, 8 (4.1%) showed metastatic disease at the radiological assessment after the signature of the informed consent and were excluded from the study before blood collection; therefore, these pts have not been considered in this analysis. Finally, preoperative VEGF-C serum levels were determined in non-consecutive 186 pts affected by non-metastatic GC observed at our department and 82 healthy people. The last follow-up time was April 28, 2017. Pts characteristics are summarized in Table 1. The median age was 61.5 years old (range, 22–88). The majority of pts were male (67.44%), presented an ECOG performance status of 0 (81.2%) and tumor located in the body of the stomach (42%). One hundred forty-five (78%) and 41 patients (22%) underwent total and subtotal gastrectomy, respectively, and the majority of pts received a D2 lymphadenectomy (67.2%); all pts underwent R0 resection with a median lymph nodes ratio (LNF ratio)—defined as the number of positive nodes divided by total nodes harvested—of 0.2. After the surgery, 144 (77.4%) pts received an adjuvant chemotherapy based on fluoropyrimidine schedule alone or in association with oxaliplatin for 6 months. Of the 42 pts (22.5%) who did not receive an adjuvant treatment, 34 pts (80.9%) underwent exclusive follow-up according to the tumor stage, whereas 8 pts (19.1%) candidates for treatment did not receive it due to post-surgery complications requiring a prolonged hospitalization.
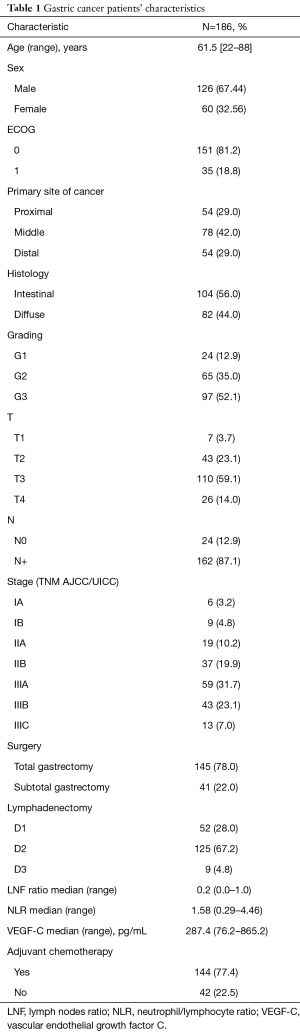
Full table
Preoperative VEGF-C serum levels were significantly higher in GC pts (median: 287.4 pg/mL; range, 76.2–865.2 pg/mL) compared with the control group (median VEGF-C: 31 pg/mL; range, 12–97 pg/mL). As already reported, we considered the median value of 287.4 pg/mL as the cut-off for our analyses.
Analysis related to pathological variables
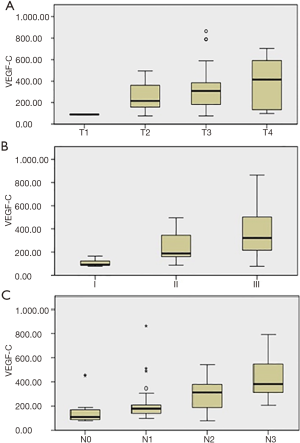
The differences in serum VEGF-C levels and their correlation with tumor variables in GC pts group are summarized in Table 2. No correlation has been found among preoperative VEGF-C levels, gender, age, ECOG and neutrophils/lymphocytes ratio (NLR, defined as the ratio between neutrophils and lymphocytes in the peripheral venous blood). On the other hand, there was a significant relationship among serum VEGF-C levels, T, N and tumor stage, showing that the protein level increases when T value, N value or stage increases (P=0.031, P=0.001, P<0.0001, respectively), while there was no significant correlation with the others clinic-pathologic variables analyzed (primary tumor site, grading and histology) (Figure 1). In particular, multiple comparison analysis showed that: pts with a pT3-T4 tumor had higher levels of the protein than those with a pT1 tumor (P=0.035 and 0.039, respectively); pts with lymph node metastasis N2-N3 had higher VEGF-C levels than pts with N0-N1 (N0-N2: P=0.005; N0-N3: P<0.001; N1-N3: P<0.001). Finally, pts with early stage tumor had lower protein levels than II and III stage (I-II: P=0.40; I-III: P<0.001; II-III: P=0.14).
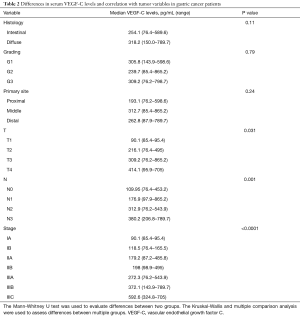
Full table
Analysis related to OS and DFS
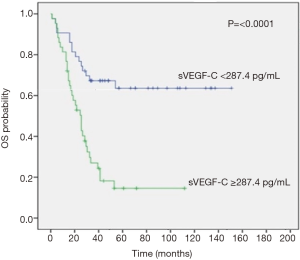
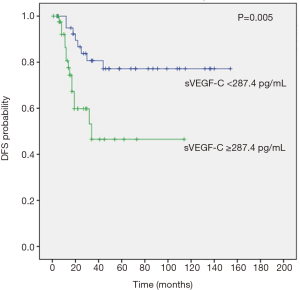
After a median follow-up of 68 months (95% CI, 51.7–84.2), all pts completed follow-up program until death from any cause without pts lost to follow-up. Median OS was significantly lower in pts with high VEGF-C levels than in pts with normal VEGF-C value [not reached (NR) vs. 26 months (95% CI, 19.9–32 months), respectively; P<0.0001] (Figure 2). Elevated preoperative VEGF-C levels correlate also with earlier disease relapse and a poor median DFS (P=0.005) and in each subgroup the median value was NR (Figure 3).
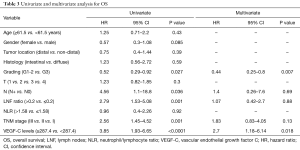
To evaluate the prognostic value of clinicopathologic variables, we performed a univariate analysis for OS and DFS, as shown in Tables 3,4. In this analysis, tumor grading [hazard ratio (HR) =0.52; 95% CI, 0.29–0.92, P=0.027), lymph node metastasis (HR =4.56; 95% CI, 1.1–18.8, P=0.036), LNF ratio (>0.2 vs. ≤0.2: HR =2.79; 95% CI, 1.53–5.08, P=0.001), tumor stage (HR =2.56; 95% CI, 1.45–4.52, P=0.001) and preoperative serum VEGF-C levels (≥287.4 vs. <287.4: HR =3.85; 95% CI, 1.93–6.65, P<0.0001) were prognostic factor that influence OS.
Full table
Full table
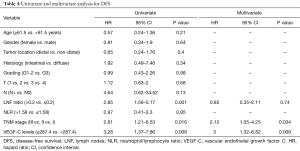
Tumor stage, LNF ratio and preoperative serum VEGF-C levels were found as prognostic factors for DFS (Table 4).
Finally, high VEGF-C levels (HR =2.7; 95% CI, 1.18–6.14; P=0.018) and tumor grading (G1-2 vs. G3: HR =0.44; 95% CI, 0.25–0.8; P=0.007) for OS and stage (HR =2.12; 95% CI, 1.05–4.25; P=0.034) and serum VEGF-C levels (HR =3; 95% CI, 1.32–6.82; P=0.008) for DFS were independent prognostic factors at multivariate analysis (Tables 3,4).
Discussion
No validated predictive and prognostic biomarkers are available for GC still today, even if the study of tumor lymph nodes spread is becoming relevant, because this process represents an early and common step in the natural development of GC. In the lymphangiogenesis process, VEGFs, in particular VEGF-C, play a key role, because they stimulate growth, angiogenesis and metastasis (25,26). In the current study, we analyzed the correlation between the preoperative circulating levels of VEGF-C, the pathological parameters of the tumor and the clinical outcome in pts with resectable GC.
We observed that serum VEGF-C levels were significantly higher in pts affected by GC than in the healthy controls and, in the first group, pts with advanced GC showed an increased value of the circulating protein. These findings are in agreement with available literature data that showed an association between VEGF-C values and the increase of T, N and tumor stage (27). Moreover, in the last two decades, different but limited trials have already investigated the prognostic role of VEGF-C overexpression in pts affected by GC, through the study of the immunohistochemical (IHC) expression in the tissue or of the levels of VEGF-C soluble forms in the serum. Nevertheless, whereas some studies and meta-analysis (27) showed that the overexpression of the protein leads to tumor growth, lymph node metastasis and a poor prognosis in term of OS (28), others refused these results. The more robust evidence comes from studies that examined the expression of VEGF-C in tissue by IHC analysis and its relationship with lymphatic vessel density (29,30). In particular, Cao et al. (31) analyzed in a meta-analysis data tissue of 1,813 pts in 13 studies, showing an important role of VEGF-C on OS in GC, but not in DFS. On these bases, nowadays the prognostic role of VEGF-C in GC remains unclear (29,30).
In this study, we observed that median OS and DFS were significantly lower in pts with high VEGF-C levels than in pts with normal VEGF-C value, suggesting that elevated preoperative protein levels correlate with earlier relapse of disease and poor prognosis. To evaluate the prognostic value of clinicopathologic variables, we performed a univariate analysis for OS and DFS, showing that tumor grading, lymph node metastasis, LNF ratio, tumor stage and preoperative serum VEGF-C levels were prognostic factor for OS as well as tumor stage, LNF ratio and preoperative serum VEGF-C levels for DFS. Subsequently, we performed a multivariate Cox regression analysis of these statistical significant parameters for OS and DFS, revealing that only elevated serum VEGF-C levels and tumor grading for OS as well as stage and serum VEGF-C levels for DFS were independent prognostic factors in pts with resectable GC.
In these results, the role of LNF ratio it should be note. In fact, if VEGF-C promotes lymphatic spread binding its receptor VEGFR3 and lymph node metastasis (32), it would expect to find LNF ratio as statistically significant in multivariate analysis. In our result, instead, we found that LNF ratio influences significantly OS and DFS only in the univariate analysis, while it is not an independent factor at the multivariate one. The explanation for this result could be found in the value 0.2 that we used as cut-off for this parameter, which represent the median value of LNF ratio. This value, indeed, is quite low, may be due to the improvement of surgery with high percentage of D2 lymphadenectomy in this study as well as the high number of lymph nodes harvested, resulting in a lower value. On the other hand, by Kruskal-Wallis and multiple comparison analysis, we found that serum VEGF-C level increases in pts with N2-N3 metastasis, as showed in Table 2 and Figure 1C. These findings confirm that serum VEGF-C levels were strongly associated with more advanced regional lymph node metastasis in pts with resected GC even after a long follow-up time. This large observation time could be considered a strength of the study.
Finally, in our study we must consider some limitations: first, our data were based on a single institution experience that did not include the analysis of tissue VEGF expression, in contrast to the majority of the data in the literature; then, we did not comprised the postoperative determination of serum VEGF-C level in the design of this study. Moreover, even if the sample size is higher than the sample size of many trials in literature, we believe that a study that involves a lager cohort of pts is needed to confirm these data and to establish the real prognostic value of serum VEGF-C in GC.
Conclusions
The present study revealed that increased serum VEGF-C levels were strongly associated with more advanced regional lymph node metastasis in pts with resected GC, appearing a poor prognostic factor correlated with nodal involvement and worse OS and DFS. However, the validation of the role of preoperative serum VEGF-C level as prognostic biomarker in pts affected by non-metastatic GC requires further studies.
Acknowledgements
We thank all patients and their families for the participation in this study.
Footnote
Conflicts of Interest: F Ciardiello: Advisory Boards: Roche, Amgen, Merck, Pfizer, Sanofi, Bayer, Servier, BMS, Celgene, Lilly; Institutional Research Grants: Bayer, Roche, Merck, Amgen, AstraZeneca, Takeda; F De Vita: Advisory Boards: Roche, Amgen, Celgene, Lilly; M Orditura: Honoraria from Italfarmaco, EISAI, epionpharma, Roche; A Petrillo: honoraria from Lilly. The authors declare that all these conflicts of interest are not connected with the issue of this paper. The other authors have no conflicts of interest to declare.
Ethical Statement: The protocol was approved by the institutional board at the center and the study was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice. All pts provided a written informed consent before the enrollment in the study.
References
- Novotny AR, Schuhmacher C. Predicting lymph node metastases in early gastric cancer: radical resection or organ-sparing therapy? Gastric Cancer 2008;11:131-3. [Crossref] [PubMed]
- Kwee RM, Kwee TC. Predicting lymph node status in early gastric cancer. Gastric Cancer 2008;11:134-48. [Crossref] [PubMed]
- Kunisaki C, Takahashi M, Nagahori Y, et al. Risk factors for lymph node metastasis in histologically poorly differentiated type early gastric cancer. Endoscopy 2009;41:498-503. [Crossref] [PubMed]
- Ishikawa S, Togashi A, Inoue M, et al. Indications for EMR/ESD in cases of early gastric cancer: relationship between histological type, depth of wall invasion, and lymph node metastasis. Gastric Cancer 2007;10:35-8. [Crossref] [PubMed]
- Galizia G, Lieto E, De Vita F, et al. Modified versus standard D2 lymphadenectomy in total gastrectomy for nonjunctional gastric carcinoma with lymph node metastasis. Surgery 2015;157:285-96. [Crossref] [PubMed]
- Zhao BW, Chen YM, Jiang SS, et al. Lymph Node Metastasis, a Unique Independent Prognostic Factor in Early Gastric Cancer. PLoS One 2015;10:e0129531. [Crossref] [PubMed]
- Song W, Yuan Y, Wang L, et al. The prognostic value of lymph nodes dissection number on survival of patients with lymph node-negative gastric cancer. Gastroenterol Res Pract 2014;2014:603194. [Crossref] [PubMed]
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [Crossref] [PubMed]
- Deng J, Liang H, Sun D, et al. The prognostic analysis of lymph node-positive gastric cancer patients following curative resection. J Surg Res 2010;161:47-53. [Crossref] [PubMed]
- Joukov V, Kaipainen A, Jeltsch M, et al. Vascular endothelial growth factors VEGF-B and VEGF-C. J Cell Physiol 1997;173:211-5. [Crossref] [PubMed]
- Oh SJ, Jeltsch MM, Birkenhäger R, et al. VEGF and VEGF-C: specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev Biol 1997;188:96-109. [Crossref] [PubMed]
- Joukov V, Pajusola K, Kaipainen A, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J 1996;15:1751. [Crossref] [PubMed]
- Kaipainen A, Korhonen J, Mustonen T, et al. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A 1995;92:3566-70. [Crossref] [PubMed]
- Su JL, Yen CJ, Chen PS, et al. The role of the VEGF-C/ VEGFR-3 axis in cancer progression. Br J Cancer 2007;96:541-5. [Crossref] [PubMed]
- Bunone G, Vigneri P, Mariani L, et al. Expression of angiogenesis stimulators and inhibitors in human thyroid tumors and correlation with clinical pathological features. Am J Pathol 1999;155:1967-76. [Crossref] [PubMed]
- Tsurusaki T, Kanda S, Sakai H, et al. Vascular endothelial growth factor-C expression in human prostatic carcinoma and its relationship to lymph node metastasis. Br J Cancer 1999;80:309-13. [Crossref] [PubMed]
- Su JL, Yang PC, Shih JY, et al. The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell 2006;9:209-23. [Crossref] [PubMed]
- Ueda M, Terai Y, Yamashita Y, et al. Correlation between vascular endothelial growth factor-C expression and invasion phenotype in cervical carcinomas. Int J Cancer 2002;98:335-43. [Crossref] [PubMed]
- Nishida N, Yano H, Komai K, et al. Vascular endothelial growth factor C and vascular endothelial growth factor receptor 2 are related closely to the prognosis of patients with ovarian carcinoma. Cancer 2004;101:1364-74. [Crossref] [PubMed]
- Gao P, Zhou GY, Zhang QH, et al. Lymphangiogenesis in gastric carcinoma correlates with prognosis. J Pathol 2009;218:192-200. [Crossref] [PubMed]
- Kigure W, Fujii T, Sutoh T, et al. The association of VEGF-C expression with tumor lymphatic vessel density and lymph node metastasis in patients with gastric cancer and gastrointestinal stromal tumor. Hepatogastroenterology 2013;60:277-80. [PubMed]
- Chen H, Guan R, Lei Y, et al. Lymphangiogenesis in Gastric Cancer regulated through Akt/mTOR-VEGF-C/VEGF-D axis. BMC Cancer 2015;15:103. [Crossref] [PubMed]
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Sobin LH, Gospodarowicz M, Wittekind C. TNM Classification of Malignant Tumours. Available online: https://www.uicc.org/sites/main/files/private/TNM_Classification_of_Malignant_Tumours_Website_15%20MAy2011.pdf
- McColm JR, Geisen P, Hartnett ME. VEGF isoforms and their expression after a single episode of hypoxia or repeated fluctuations between hyperoxia and hypoxia: relevance to clinical ROP. Mol Vis 2004;10:512-20. [PubMed]
- Melnyk O, Shuman MA, Kim KJ. Vascular endothelial growth factor promotes tumor dissemination by a mechanism distinct from its effect on primary tumor growth. Cancer Res 1996;56:921-4. [PubMed]
- Karayiannakis AJ, Syrigos KN, Polychronidis A, et al. Circulating VEGF Levels in the Serum of Gastric Cancer Patients Correlation With Pathological Variables, Patient Survival, and Tumor Surgery. Ann Surg 2002;236:37-42. [Crossref] [PubMed]
- Wang X, Chen X, Fang J, et al. Overexpression of both VEGF-A and VEGF-C in gastric cancer correlates with prognosis, and silencing of both is effective to inhibit cancer growth. Int J Clin Exp Pathol 2013;6:586-97. [PubMed]
- Xie LX, Zhai TT, Yang LP, et al. Lymphangiogenesis and Prognostic Significance of Vascular Endothelial Growth Factor C in Gastro-oesophageal Junction Adenocarcinoma. Int J Exp Pathol 2013;94:39-46. [Crossref] [PubMed]
- Wang TB, Deng MH, Qiu WS, et al. Association of serum vascular endothelial growth factor-C and lymphatic vessel density with lymph node metastasis and prognosis of patients with gastric cancer. World J Gastroenterol 2007;13:1794-7. [Crossref] [PubMed]
- Cao W, Fan R, Yang W, et al. VEGF-C expression is associated with the poor survival in gastric cancer tissue. Tumour Biol 2014;35:3377-83. [Crossref] [PubMed]
- Jüttner S, Wissmann C, Jons T, et al. Vascular endothelial growth factor-D and its receptor VEGFR-3: two novel independent prognostic markers in gastric adenocarcinoma. J Clin Oncol 2006;24:228-40. [Crossref] [PubMed]


