
Trends in surgery and disparities in receipt of surgery for intrahepatic cholangiocarcinoma in the US: 2005–2014
Introduction
Cholangiocarcinoma (CCA) comprises a heterogeneous group of cancers with pathologic features of biliary tract differentiation and is presumed to arise from the intra- or extrahepatic biliary tract. CCA is best classified anatomically as intrahepatic, perihilar, or distal CCA (1,2). The incidence of CCA varies substantially worldwide with the highest known rates in Northeast Thailand (>80 per 100,000) and much lower rates in the Western world, for example Canada (0.3 per 100,000) (3). Intrahepatic cholangiocarcinoma (IHC) is the second most common primary liver cancer in humans, after hepatocellular carcinoma (HCC) (4), although the frequency is considerably less than HCC. Several recent studies from around the world have reported rapidly rising incidence and mortality over the last few decades (5-8). A recent SEER database study over a 40 year period demonstrated an annual percentage change of 2.30% or a 128% increase over that time period (9). However it is unclear if this is a true increase or a result of misclassification due to the change in International Classification of Disease (ICD) coding (10).
The mainstay of treatment for IHC is surgery with a reported 5-year survival of up to 40% in patients who have had a curative resection (R0 resection). However, few patients are candidates for surgery because most present with advanced disease (11,12). There have been numerous advancements in liver surgery that have expanded the indications for resection of metastatic and primary liver malignancies (13). Despite this evolution in liver surgery, the use of surgery for the treatment of IHC has not been well characterized on a representative, nationwide basis. The objective of this study is to utilize a nationwide representative database to quantify the trends in IHC related hospitalizations, and surgery procedures in the US from 2005–2014 as well as identify any disparities in the receipt of surgery during this time.
Methods
Study approval was obtained from the Western Michigan University Homer Stryker MD School of Medicine Institutional Review Board (WMed IRB) and considered as an exempt non-human subject study. We retrospectively analyzed the Nationwide Inpatient Sample (NIS) data from 2005 to 2014 to examine hospitalization trends, length of stay and cost of hospitalization for patients with the diagnosis of IHC in US hospitals. The NIS is the largest all-payer database suitable for analysis of trends in health care utilization, access, charges, quality, and outcomes for both research and policy-making purposes (HCUP databases). We identified all cases of IHC admissions in the NIS database over ten years period, using the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9) coding. Patients with IHC related admissions were identified using ICD-9 code of 155.1 (malignant neoplasm of intrahepatic bile ducts). The data was weighted to represent the entire inpatient population with IHC. Using the procedure code in the database, we determined all IHC directed surgical procedures. The procedures of interest include; partial hepatectomy (50.22), hepatic lobectomy (50.3), hepatic wedge resection (50.12). We stratified procedures into two groups—major surgical procedure (hepatic lobectomy) and grouped other procedures as minor surgical procedures.
We also extracted data on patient demographics, hospital characteristics (teaching or nonteaching status and urban versus rural), length of hospital stay (LOS), total hospital charge, sources of primary payment (patient insurance status), and mortality. Elixhauser index scores were calculated to account for comorbidities.
Statistical methods
Statistical analyses were performed using SAS version 9 (SAS Institute, Cary, North Carolina). The total number of admissions for IHC in the study population was determined for each year from 2005–2014. Admission rate for IHC was calculated as the yearly total of IHC related admissions divided by the total number of admission for each corresponding year (reported as per 100,000 admissions per year). Annual percentage change (AAPC) and corresponding 95% confidence interval (CI) for IHC hospitalization and number of surgical procedures for IHC was determined from 2005 to 2014. Descriptive statistics were used to report frequencies and percentages for categorical variables and group differences were compared with Chi-square test. Continuous variables were reported as means with standard deviation (SD) or medians with interquartile range (IQR), as appropriate. Continuous variables were compared with independent t-test or Mann-Whitney test. Finally, we performed a multivariable regression analyses to examine factors associated with inpatient IHC surgical procedures and reported parameters as adjusted odds ratio and 95% confidence intervals. P values <0.05 were considered statistically significant.
Results
Trend in IHC related hospitalizations
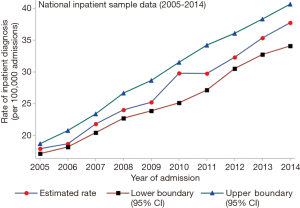
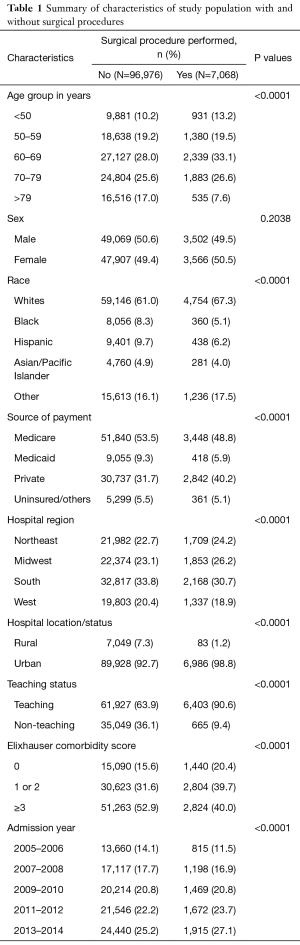
Between 2005 and 2015, there were 20,978 (104,045 after weighting) admissions coded as being due to IHC. The number of recorded admissions increased from 1,435 (7,097 after weighting) in 2005 to 2,754 (13,770 after weighting) in 2014, which approximates to nearly a 2-fold increase in admission rate for IHC of 38.9 per 100,000 in 2014 (95% CI, 35.7–42.2) from 18.1 per 100,000 (95% CI, 15.8–20.3) in 2005 (Figure 1). The AAPC for IHC admission increased remarkably from 3.9% (95% CI, 3.7–4.1%) to 7.4% (95% CI, 6.6–8.3%). There was an increasing trend seen across races including White, Black and Hispanic. However, no remarkable difference in hospitalization was seen between males and females. The mean (SD) age of patients hospitalized for IHC decreased significantly from 69 years (±13) in 2005 to 65 years (±12) in 2014 (P value <0.0001). Majority of the patients admitted for IHC were 60 years or older, Caucasians, and had Medicare as the primary source of payment. About ninety-three percent of admissions occurred in urban hospitals and 64% were in teaching hospitals (Table 1).
Full table
Trends in surgery
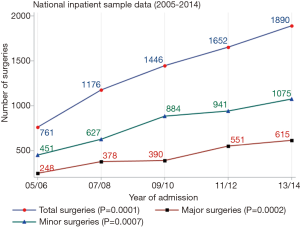
Figure 2 depicts the trends in inpatient surgery procedures for IHC. The total number of IHC related surgical resections increased steadily from 761 resections in 2005 to 1,890 resections, representing a 248.3% increase in the number of resections. This approximates to an increase AAPC in surgical resection of 0.6% (95%, CI, 0–6.3%) to 9.8% (95%, CI, 8.3–12.7%) in 2015. Majority of total resections were minor resections (56%). During the study period there were no observed difference in the age and sex of patients undergoing resection. However, there were differences the number of resections by race, hospital location (rural vs. urban), and teaching status. Percentage of Caucasian patients who underwent resection increased form 54.7% to 73.4% (P<0.05). Majority of surgeries were performed in teaching hospitals which increased from 86.7% in 2005/2006 to 92.7% in 2013/2014 (P<0.05). There was also an increase in the proportion of surgeries being performed in urban hospitals compared to rural hospital locations.
Trend in length of stay, mortality, and cost
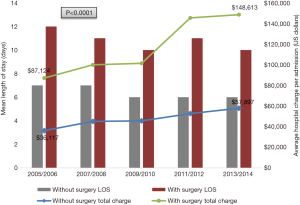
Overall, the LOS and inpatient mortality for IHC related admission decreased significantly over overtime. However, a remarkable increase in the cost for inpatient care for IHC increased remarkably (Figure 3). Although no statistically significant difference in mortality trend was observed among patients who had surgical procedure (Figure 4), there were differences in mortality, LOS and hospitalization cost based on location of the hospital or teaching status. Compared to rural hospitals, Urban hospital admissions had significantly higher mean LOS (7 vs. 5 days) and cost of inpatient care ($56,175 vs. $22,684) and lower mortality was also observed in urban location compared to rural (8.2% vs. 10.3%, P=0.0050). The percentage mortality of admitted patients with IHC was higher among those who had major surgical procedure than minor surgical (6% vs. 7.7%). Compared to non-teaching hospital admissions, fewer mortality cases were observed in teaching hospitals (7.7% vs. 9.6%) during the study period. Nevertheless, the mean LOS and cost for hospitalization among IHC patients who had surgical procedures were significantly higher among admissions in teaching hospitals than non-teaching hospitals, with LOS of 11 vs. 10 days and $124,278 vs. $107,551 respectively (P values <0.05).
Receipt of surgery
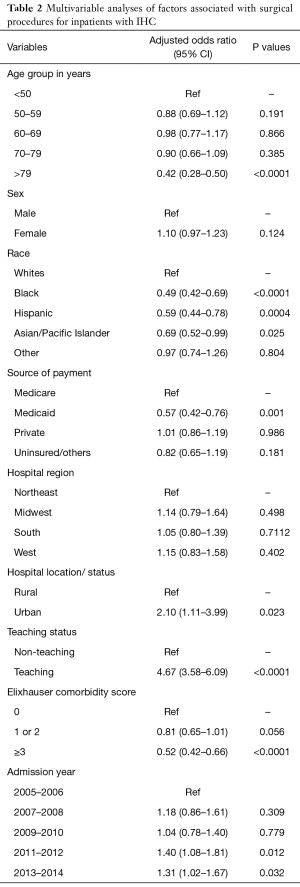
Of the total 104,044 admissions recorded for IHC, 7,068 patients underwent surgery, representing approximately 6.8% of the total number of IHC admissions. Patients who underwent liver resection were younger, more likely to be Caucasian, have private insurance, and a lower comorbidity index score. Surgery procedures were four times more likely to be performed in teaching hospitals compared to non-teaching (AOR, 4.67; 95% CI, 3.58–6.09) and twice likely to performed in urban versus rural located hospitals (AOR, 2.10; 95% CI, 1.11–3.99) (Table 2). Patients who had liver resection procedures had longer mean LOS than no open surgery (11 vs. 7 days) and higher hospitalization cost ($129,965 vs. $49,106, P<0.05).
Full table
To assess the magnitude of observed differences between patients who had liver resection, predictors of liver resection were weighted in a multivariate analysis. Patients younger than 50 years had a 2-fold greater likelihood of undergoing surgery. Patients with a lower comorbidity score were more likely to have surgery. Resections were less likely to be performed in African Americans, Hispanics and Asians. Medicaid patients were also less likely to have surgery. Resections were more likely performed in teaching and urban hospitals.
Discussion
Although many authors have reported an increasing incidence of IHC, there has been a debate as to the veracity of studies given the classification changes in the WHO ICD-O coding system between 1992 and 2000 (14-16). Saha et al. using the SEER database correcting for coding errors demonstrated increases over both recent [2003–2013] and long term time periods [1973–2012] associated with an AAPC of 4.36% and 2.30% respectively (9). Our study analyzing NIS data over the last decade has demonstrated a concomitant increase in hospitalizations for patients with a diagnosis of IHC from 18.1 per 100,000 (95% CI, 15.8–20.3) in 2005 to 38.9 per 100,000 (95% CI, 35.7–42.2) in 2014. This data further supports the previously reported data that incidence rates of IHC are increasing. Therefore, presumably more patients are being hospitalized with a diagnosis of IHC. This increase may also be due to our improved ability to recognize and diagnose IHC as well as increasing rates of diabetes, obesity, alcohol use and viral hepatitis, factors which have all been associated with IHC (17).
Liver surgery has evolved significantly over the past decades with documented increases in the number of patients undergoing surgery for both benign and malignant diseases (18,19). IHC is associated with poor prognosis and the primary determinant of long term survival is one’s ability to undergo resection (12,20). However, it is unclear if the documented increases in the incidence of IHC have resulted in an increase in surgery for IHC. There have been single institution reports of increased frequency of surgery for IHC over time notably from Memorial Sloan Kettering Cancer Center, where an annual percentage increase of 14% between 1990 and 2006 (21) was reported. This study utilizing a national database also demonstrated an overall increase in patients having surgery for IHC. This increase represents a 248% increase. There were notable demographic differences in the patients who had surgery over time. In particular, the percentage of Caucasian patients having surgery over time increased from 54.7% to 73.4% (P<0.05), without an observed increase in hospitalizations. The reason for this increase is unclear, however, it might be due to the changing epidemiology of the disease with reports of higher increases in rates of IHC in Caucasians and African Americans compared to other races (22). The percentage of surgeries done at urban teaching hospital has also increased over time which is similar to reported trends in hepatobiliary and pancreatic surgery (13,23,24).
Numerous studies have reported improved perioperative outcomes such as LOS and mortality following hepatobiliary and pancreatic surgery over time (25). As expected the LOS decreased over the study time. This is likely multifactorial. Emergence of ERAS and other institutional procedure-specific pathways have been shown to decrease LOS. Interestingly, the mortality associated with surgery for IHC did not change over the time period. Dramatic changes in mortality following HPB surgery occurred in previous decades and may be the reason as to why significant changes have not been seen in our analysis of recent data (13). Some of the improvements associated with regionalization were less likely accrued in IHC surgery given the high proportion of patients who had resections at teaching hospital throughout the study period. Lower mortality was observed in teaching and urban hospitals compared to non-teaching and rural hospital respectively. Cost as expected increased throughout the study period.
Surgery remains the only treatment modality associated with long term survival in patients with IHC (26). However, only a small majority of patients are candidates for surgery (27). In this study we evaluated the predictors of receipt of surgery in patients hospitalized with IHC. As expected a higher comorbidity score was associated with a decreased likelihood of having surgery. Race and insurance also appeared to be factors in the receipt of surgery. These disparities in race and insurance have been and continue to be reported for a variety of different cancer diagnosis but have not been evaluated in IHC (28-30). Recent reports utilizing the SEER database have also reported disparities in survival based on race and income in patients with IHC (22). Further studies are warranted to determine if differences in treatment account for these disparities.
Our study has several limitations. First, the NIS database records inpatient procedures, which, while sufficient, does not provide as complete a picture as including outpatient procedures would. Additionally, the NIS database does not collect patient-specific data on key prognostic factors such as tumor burden and stage, any bile duct abnormalities such as whether any degree of biliary cholangitis were present, and whether there was any degree of associated cirrhosis. These prognostic factors play a key role not only in appropriateness of surgery but consequent outcomes in mortality and cost. The use of inpatient data without unique identifiers also allows for the possibility of a given individual patient being recorded multiple times. Finally, this study describes trends in the use of surgical therapy for IHC and cannot conclude the reasons for these trends or assess the appropriateness of therapies used. Therefore, disparities in receipt of surgery may arise from other variables not captured by the database such as those previously mentioned.
Conclusions
There has been an increase in the hospitalization of patients with a diagnosis of IHC in the past decade. This further suggests that the reported rising incidence in IHC is being reflected as increasing inpatient admissions. There has also been a consistent increase in the number of patients undergoing surgery. Importantly there are observed racial and socioeconomic disparities that influence the receipt of surgery for patients with IHC. Further studies utilizing registries with detailed information on stage and patient specific variables are critical to assess disparities in receipt of surgery and provide better understanding of this association.
Acknowledgements
None.
Footnote
Conflicts of Interest: Presented at the SSAT’s 59th Annual Meeting at Digestive Disease Week, June 2-5, 2018, in Washington, DC.
Ethical Statement: Study approval was obtained from the Western Michigan University Homer Stryker MD School of Medicine Institutional Review Board (WMed IRB) and considered as an exempt non-human subject study.
References
- Blechacz B, Komuta M, Roskams T, et al. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2011;8:512-22. [Crossref] [PubMed]
- Cholangiocarcinoma Patel T. Nat Clin Pract Gastroenterol Hepatol 2006;3:33-42. [Crossref] [PubMed]
- Shaib Y, El-Serag H. The Epidemiology of Cholangiocarcinoma. Semin Liver Dis 2004;24:115-25. [Crossref] [PubMed]
- Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet 2005;366:1303-14. [Crossref] [PubMed]
- West J, Wood H, Logan RF, et al. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971-2001. Br J Cancer 2006;94:1751-8. [Crossref] [PubMed]
- Lee TY, Lin JT, Kuo KN, et al. A nationwide population-based study shows increasing incidence of cholangiocarcinoma. Hepatol Int 2013;7:226-32. [Crossref] [PubMed]
- Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB 2008;10:77-82. [Crossref] [PubMed]
- Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2002;2:10. [Crossref] [PubMed]
- Saha SK, Zhu AX, Fuchs CS, et al. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016;21:594-9. [Crossref] [PubMed]
- Tyson GL, Ilyas JA, Duan Z, et al. Secular Trends in the Incidence of Cholangiocarcinoma in the USA and the Impact of Misclassification. Dig Dis Sci 2014;59:3103-10. [Crossref] [PubMed]
- Lee JS, Hu HM, Edelman AL, et al. New Persistent Opioid Use Among Patients With Cancer After Curative-Intent Surgery. J Clin Oncol 2017;35:4042-9. [Crossref] [PubMed]
- Tan JC, Coburn NG, Baxter NN, et al. Surgical Management of Intrahepatic Cholangiocarcinoma - A Population-Based Study. Ann Surg Oncol 2008;15:600-8. [Crossref] [PubMed]
- Dimick JB, Wainess RM, Cowan JA, et al. National trends in the use and outcomes of hepatic resection. J Am Coll Surg 2004;199:31-8. [Crossref] [PubMed]
- Welzel TM, Graubard BI, El-Serag HB, et al. Risk Factors for Intrahepatic and Extrahepatic Cholangiocarcinoma in the United States: A Population-Based Case-Control Study. Clin Gastroenterol Hepatol 2007;5:1221-8. [Crossref] [PubMed]
- Khan SA, Emadossadaty S, Ladep NG, et al. Rising trends in cholangiocarcinoma: Is the ICD classification system misleading us? J Hepatol 2012;56:848-54. [Crossref] [PubMed]
- Khan SA, Taylor-Robinson SD, Toledano MB, et al. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol 2002;37:806-13. [Crossref] [PubMed]
- Petrick JL, Campbell PT, Koshiol J, et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The Liver Cancer Pooling Project. Br J Cancer 2018;118:1005-12. [Crossref] [PubMed]
- Kim Y, Amini N, He J, et al. National trends in the use of surgery for benign hepatic tumors in the United States. Surgery 2015;157:1055-64. [Crossref] [PubMed]
- Dimick JB, Wainess RM, Cowan JA, et al. National trends in the use and outcomes of hepatic resection1 1No competing interests declared. J Am Coll Surg 2004;199:31-8. [Crossref] [PubMed]
- Benson AB, D’Angelica MI, Abbott DE, et al. NCCN Guidelines Insights: Hepatobiliary Cancers, Version 1.2017. J Natl Compr Canc Netw 2017;15:563-73. [Crossref] [PubMed]
- Endo I, Gonen M, Yopp AC, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 2008;248:84-96. [Crossref] [PubMed]
- Mukkamalla SKR, Naseri HM, Kim BM, et al. Trends in Incidence and Factors Affecting Survival of Patients With Cholangiocarcinoma in the United States. J Natl Compr Canc Netw 2018;16:370-6. [Crossref] [PubMed]
- Stitzenberg KB, Sigurdson ER, Egleston BL, et al. Centralization of cancer surgery: implications for patient access to optimal care. J Clin Oncol 2009;27:4671-8. [Crossref] [PubMed]
- O’Mahoney PR, Yeo HL, Sedrakyan A, et al. Centralization of pancreatoduodenectomy a decade later: Impact of the volume-outcome relationship. Surgery 2016;159:1528-38. [Crossref] [PubMed]
- Gani F, Thompson VM, Bentrem DJ, et al. Patterns of hepatic resections in North America: use of concurrent partial resections and ablations. HPB (Oxford) 2016;18:813-20. [Crossref] [PubMed]
- Hyder O, Marques H, Pulitano C, et al. A Nomogram to Predict Long-term Survival After Resection for Intrahepatic Cholangiocarcinoma. JAMA Surg 2014;149:432. [Crossref] [PubMed]
- Ejaz A, Spolverato G, Bridges JF, et al. Choosing a Cancer Surgeon: Analyzing Factors in Patient Decision Making Using a Best–Worst Scaling Methodology. Ann Surg Oncol 2014;21:3732-8. [Crossref] [PubMed]
- Munene G, Parker RD, Shaheen AA, et al. Disparities in the surgical treatment of colorectal liver metastases. J Natl Med Assoc 2013;105:128-37. [Crossref] [PubMed]
- Bilimoria KY, Bentrem DJ, Ko CY, et al. National Failure to Operate on Early Stage Pancreatic Cancer. Ann Surg 2007;246:173-80. [Crossref] [PubMed]
- Murphy MM, Simons JP, Hill JS, et al. Pancreatic resection. Cancer 2009;115:3979-90. [Crossref] [PubMed]



