
Radiation therapy for rectal cancer
Introduction
In 2018, 1.8 million people worldwide were diagnosed with colorectal cancer and 881,000 deaths from it were expected. It is the third most common cancer worldwide and second most common cause of cancer mortality (1). One third of colorectal cancers involve the rectum. A multidisciplinary approach is used in the treatment of rectal cancer which involves the three modalities of cancer therapy: surgery, chemotherapy and radiotherapy (RT).
RT in early stage rectal cancer
Endocavitary radiation
In 1973, Papillon proposed the use of endocavitary radiation for the treatment of early stage rectal cancers. The procedure involved the use of a specialised proctoscope which permitted the passage of an X-ray tube, placed in direct contact with the tumour. Tumours suitable for this treatment were usually small, T1 or T2 tumours. Three to five applications lasting 2–4 minutes each were applied to the tumour over 4–6 weeks. It was well tolerated by elderly, frail patients and could be done as an outpatient (2). Their analysis of 312 patients found that 5-year local and nodal failure rates were 4.5% and 3.8%, respectively. The rate of death from cancer was 7.7% (3).
Despite the advantages of endocavitary radiation being non-invasive, well tolerated and with good outcomes, few centres around the world have perpetuated this technique. This is largely due to the advancement of newer surgical techniques such as Trans-anal Endoscopic Micro Surgery (TEMS) and Trans Anal Minimally Invasive Surgery (TAMIS) which are currently offered to patients with early-stage rectal cancers (4).
Preoperative chemo-RT followed by local excision
ACOSOG Z6041 was a multi-institutional prospective study that investigated the outcomes of preoperative chemo-RT and local excision in patients with early-stage (T2N0) rectal cancers. At a median follow-up of 56 months, 3-year disease-free survival was 88.2% in the intention-to-treat group and 86.9% in the per-protocol group. They concluded that preoperative chemo-RT followed by local excision could be considered as an organ-preserving alternative in these early-stage tumours, but this conclusion could not be extrapolated to locally advanced tumours that had a good response (5).
Postoperative RT after local excision
The desire to maintain quality of life for rectal cancer patients led to the investigation of local excision of early-stage rectal cancers rather than radical surgery. Results from studies comparing local excision to radical surgery have been mixed and currently only clinically staged T1 tumours with favourable histology are considered for local excision alone (6).
In order to improve the oncological outcomes of local excision, the Cancer and Leukemia Group B (CALGB) conducted a prospective study looking at patients with T1 and T2 distal rectal cancer treated with local excision, where only T2 tumours received adjuvant chemo-RT. They found that T2 tumours had a higher rate of recurrence and shorter overall and disease-free survival even with adjuvant chemo-RT, when compared to T1 tumours treated with local excision alone (7).
A Korean group studied a similar group of patients (T1 or T2 rectal cancer) whom all underwent adjuvant chemo-RT after local excision in an attempt to avoid radical resection. They found the only significant factor affecting disease-free survival was tumour stage and patients with T2 tumours had inferior disease-free survival compared to T1 tumours (8).
A single hospital case-control study evaluated the efficacy of adjuvant RT alone after local excision for early rectal cancer (T1 only). The recurrence-free survival at 5 years was 96.8% in the adjuvant RT group and 97% in the local excision group (P=0.657), showing that RT may not improve recurrence-free survival compared with local excision alone (9).
Table 1 summarises the studies investigating local excision followed by RT/chemo-RT.
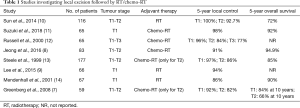
Full table
RT in locally advanced rectal cancer
Prior to the mid-1980s, surgery alone was the mainstay of treatment of locally advanced rectal cancer. This unfortunately resulted in high local recurrence rates and poor overall survival. With the advent of TME surgery, local recurrence rates were reduced to the order of 5–10% and therefore became the standard of care (15). In the 1990s, early randomised trials established surgery follow by RT alone as the standard of care for patients with T3/4, N+ rectal cancer (16). This sequence of treatment modalities improved local recurrence rates and also allowed for accurate histopathological staging, avoiding overtreatment in patients.
The National Surgical Adjuvant Breast and Bowel Project (NSABP) R-01 trial compared adjuvant chemotherapy and adjuvant RT to observation and found that adjuvant chemotherapy improved disease-free survival and overall survival compared to no further treatment, while adjuvant RT improved local recurrence rates but had no impact on disease-free survival (17).
Two randomised trials then investigated the role of adjuvant chemotherapy and found that combined modality treatment improved local recurrence rates compared to either adjuvant chemo-RT or RT given alone. Both, however, showed no difference in overall survival (18,19).
However, O’Connell et al. was able to show that the combination of a protracted infusion of fluorouracil and RT improved time to relapse and survival in a randomised trial of patients with stage 2 or 3 rectal cancer. Six hundred and sixty-six patients were randomised to concurrent intermittent bolus injections or protracted venous infusions of fluorouracil with postoperative radiation. They also received systemic chemotherapy with semustine plus fluorouracil or with fluorouracil alone in a higher dose, administered before and after RT. After a median follow-up of 46 months, patients who received the protracted infusion of fluorouracil had a significantly increased time to relapse (P=0.01) and improved survival (P=0.005) (20).
The NSABP R-02 trial sought to provide further evidence that adjuvant chemoradiation was beneficial. Three hundred and forty-six patients were randomised to adjuvant chemotherapy alone or adjuvant chemo-RT. They found that the addition of RT improved local recurrence rates but had no benefit for disease-free survival or overall survival (21).
In 1990, the National Institute of Health advocated the standard of care for locally advanced rectal cancer should be surgery followed by adjuvant chemo-RT (22).
Preoperative vs. postoperative chemo-RT
In 2004, the seminal German CAO/ARO/AIO 94 phase III trial compared preoperative to postoperative chemo-RT in 823 patients with locally advanced rectal cancer (T3 or T4 or node positive) and found significantly lower recurrence rates and acute and chronic toxicity with preoperative chemo-RT. The 5-year cumulative incidence of local recurrence was 6% and 13% in the pre- and postoperative arms respectively (23). The 10-year cumulative incidence of local recurrence was 7.1% and 10.1% in the pre- and postoperative arms, respectively (P=0.048). There were no significant differences for 10-year cumulative incidence of distant metastases and disease-free survival (24).
The NSABP-R-03 trial also compared preoperative to postoperative chemo-RT in a similar group of patients as the German trial. The 5-year disease-free survival for preoperative patients was 64.7% vs. 53.4% for postoperative patients (P=0.011). The 5-year overall survival for preoperative patients was 74.5% vs. 65.6% for postoperative patients (P=0.065). Although not statistically significant, there was a trend to improved overall survival. A complete pathologic response was achieved in 15% of preoperative patients (25).
Short course RT
In the pre-TME surgery era, the Swedish Rectal Cancer Trial recruited patients with cT1-3 rectal cancer and randomized them to receive short course RT 25 Gy/5# followed by surgery versus surgery alone. The preoperative RT group had a significant decrease in local recurrence rates (12% vs. 27%; P<0.001) and a significant improvement in 5-year overall survival (58% vs. 48%; P=0.004) (26). After 13 years, the overall survival rates are still significantly improved (38% vs. 30%; P=0.008) (27). This is the only trial investigating preoperative short course RT to show an overall survival benefit for preoperative RT.
Subsequently, the Dutch conducted a randomised trial comparing TME surgery to preoperative RT (25 Gy/5#) followed by TME surgery. There was no difference in overall survival but there was significant increased local control in the preoperative RT arm of 5.6% compared to 10.9% in the TME surgery alone arm (28).
The MRC CR07 and NCIC-CTG C016 trial compared short course preoperative RT to post-operative chemo-RT for patients with operable rectal cancer. The local recurrence rate for preoperative RT was lower at 4.4% compared to post-operative chemo-RT at 10.6%. There was a relative improvement in disease-free survival of 24% for the preoperative RT arm and overall survival did not differ between the two arms (29).
Short course RT vs. long course chemo-RT
Two options for neoadjuvant therapy prior to surgery for the management of rectal cancer are currently in use: short course RT (25 Gy/5#) or long course chemo-RT which usually consists of 50.4 Gy/28# RT with concurrent chemotherapy. In the United States of America, long course chemo-RT remains the standard of care due to concerns regarding late radiation toxicities with a hypofractionated schedule. However, in Europe, short course RT is the preferred option (30).
In 1993, the European Organisation for Research and Treatment of Cancer (EORTC) 22921 trial was published which investigated the benefit of preoperative chemo-RT versus preoperative RT alone and the benefit of additional chemotherapy versus none. A total 1,011 patients were enrolled and no significant difference was found between pre- and post-operative chemotherapy. Local control rates were 8.7%, 9.6%, and 7.6% in the groups that received chemotherapy preoperatively, postoperatively, or both, respectively, and 17.1% in the group that did not receive chemotherapy (P=0.002). The authors concluded that it was not the timing of chemotherapy that determined benefit but the mere inclusion of chemotherapy in the treatment regime that conferred benefit (31).
Compared to post-operative RT, preoperative RT has the advantage of downsizing the tumour to improve circumferential resection margins and increase the chances of a sphincter saving procedure (32). The tumour is also better oxygenated and hence increasing its radio-sensitivity to RT. The anastomosis will be unaffected postoperatively as any irradiated tissue would have been resected. However, the main disadvantage of this approach is the potential overtreatment of patients which is expected to occur in about 18% of patients (23).
Led by Bujko, the Polish group compared preoperative short course RT with conventionally fractionated chemo-RT for T3 or T4 resectable rectal cancer. There was no significant difference in overall survival, disease-free survival, local recurrence rates or late toxicity (33).
These outcomes were corroborated by the Trans-Tasman Radiation Oncology Group (TROG) 01.04 trial which also compared short course RT and long course chemo-RT and found no significant difference in the 3-year local recurrence rates of 7.5% for short course RT and 4.4% for long course chemo-RT. The 5-year overall survival rates were similar at 74% and 70% for short course RT and long course chemo-RT respectively. They found that long course chemo-RT was more effective at reducing the local recurrence for distal tumours. Late toxicity was similar for both arms (34).
We would recommend long course chemo-RT for patients with T4 tumours invading adjacent organs, threatened circumferential margins and distal tumours. Short course RT is a valid option for all other tumours.
Intensity-modulated RT (IMRT) vs. 3D conformal RT
The standard RT technique for treating rectal cancer has been 3D conformal RT, however with the advent of IMRT and its advantages of improved dose conformality allowing lower doses to organs at risk (OARs), its use has been increasing. Dosimetric studies showed that the bowel volume receiving 45–50 Gy was significantly reduced with IMRT which could potentially reduce bowel toxicity (35).
Figure 1 shows the dose distribution for 3D conformal RT and IMRT.
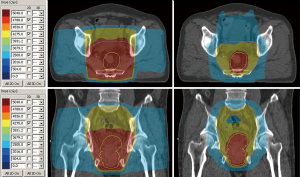
David et al. compared IMRT to 3D conformal RT when used as neoadjuvant therapy with concurrent chemotherapy for local advanced rectal cancer. They analysed 128 patients and found that IMRT was significantly associated with less toxicity and improved partial responses (36).
Tey et al. conducted a Phase 2 trial involving 23 patients with locally advanced rectal cancer treated with concurrent capecitabine and dose escalated IMRT (55 Gy in 25 fractions). Thirty-five percent of patients had a pathological complete response and 65% of patients had tumour downstaging. At a median follow-up of 38.2 months, there was no local recurrences and only one patient (5%) had grade 3 radiation proctitis (37).
Currently, the National Comprehensive Cancer Network (NCCN) recommend that IMRT should only be used in the context of a clinical trial (38), however it can be considered in patients with distal rectal/anal adenocarcinomas in whom the inguinal lymph nodes are treated. Chemo-IMRT is also a reasonable option in patients who refuse or who are not surgical candidates. RT dose escalation in these patients have been shown to be safe and associated with high rates of complete responses (37).
Dose distribution for 3D conformal radiotherapy (left) and IMRT (right). Prescription dose 50.4 Gy. IMRT, intensity-modulated radiotherapy.
High dose rate brachytherapy (HDRBT)
The use of HDRBT for rectal cancer has been studied as an alternative RT technique which would allow a highly conformal dose distribution to the rectal tumour while reducing dose to the adjacent OARs such as the bladder. Figure 2 shows the conformal dose distribution for HDRBT of the rectum.
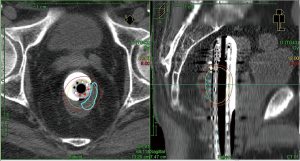
Using data from the Swedish Rectal Cancer Registry, 318 patients treated with preoperative HDRBT followed by surgery were matched to 318 patients who received preoperative short course RT and 318 patients who received no preoperative therapy. There was less perioperative bleeding in patients who underwent HDRBT and the re-intervention rate was also lower in these patients. The HDRBT group also had fewer R2 resections (39).
A systematic review analysed 12 studies which investigated preoperative HDRBT with chemo-RT or HDRBT alone. They found that the pathologic complete response rate ranged between 18% and 31%; R0 resection rate was between 80% and 99%; and sphincter-preservation rate was between 29% and 54%. The 2-year progression-free survival and overall survival rates were 68.1% and 81.5%, respectively. After preoperative HDRBT alone, the pathological complete response rate ranged between 10.4% and 27%, the R0 rate was 96.5% and the sphincter-preservation rate ranged between 53.8% and 75.8%. The 5-year progression-free survival and overall survival rates were 66.6% and 70.8%, respectively (40).
The phase 1 HERBERT study evaluated the toxicity and efficacy of HDRBT boost after external beam RT (EBRT) for elderly or medically inoperable patients with rectal cancer. They reported a 60.6% complete response rate with 2-year local progression-free survival and overall survival of 42% and 63% respectively. However, severe toxicity was observed in 10 out of 32 (31%) patients (41).
As such, HDRBT for rectal cancer is still investigational and requires further studies to support its use in clinical practice. HDR brachytherapy can be used alone, or in conjunction with EBRT for patients with rectal cancer treated with palliative intent (42).
Timing of surgery
The optimal timing of surgery after preoperative therapy has been debated. The Lyon R90-01 trial compared an interval of 2 weeks versus 6–8 weeks between completion of RT and surgery. The longer interval between preoperative RT and surgery was associated with a significantly better clinical tumour response (53.1% in the 2-week group vs. 71.7% in the 6–8-week group, P=0.007) and pathologic downstaging (10.3% in the 2-week group vs. 26% in the 6–8-week group, P=0.005). They found no difference in morbidity, local relapse, and short-term survival between the two groups (43).
Habr-Gama et al. investigated an even longer interval after neoadjuvant therapy of 12 weeks and found that a delay in surgery after neoadjuvant chemoradiotherapy is safe and does not negatively affect survival (44).
A population study of 2,094 patients showed that lengthening the interval (>13 weeks) from chemo-RT to surgery improves the pathological response, be it complete or partial (45).
GRECCAR 6 was a randomised controlled trial that compared an interval of 7 to 11 weeks from completion of neoadjuvant chemo-RT to surgery. Their primary endpoint was a pathological complete response (ypT0N0) and they found no difference between the two arms. Waiting 11 weeks was associated with a worse quality of mesorectal resection surgery (46).
Achieving a pathological complete response was an added benefit of delaying surgery and it was shown in several studies that a longer interval between neoadjuvant therapy and surgery was associated with a higher chance of achieving a pathological complete response. This, in turn, was associated with improved disease-free survival and local control (47-49).
The results of the Stockholm 3 trial were highly anticipated as it was thought to answer the question of the optimal RT fractionation and interval between RT and surgery. In this multicentre, randomised, non-blinded, phase 3, non-inferiority trial, 840 patients were randomised to receive either 5×5 Gy radiation dose with surgery within 1 week (short-course RT) or after 4–8 weeks (short-course RT with delay) or 25×2 Gy radiation dose with surgery after 4–8 weeks (long-course RT with delay). They concluded that the outcomes of delaying surgery after short-course RT were similar to short-course RT with immediate surgery. Long-course RT with delay was similar to both short-course regimens, but prolonged the treatment time substantially. Therefore, they recommended short-course RT with delay to surgery as an alternative to short-course RT with immediate surgery (50).
Is surgery necessary?
Preoperative therapy has been shown to be beneficial in terms of tumour downstaging and some patients achieve pathological complete responses. Retrospective institutional data showed that between 50–70% of patients achieve tumour or nodal downstaging and 11–13% of patients achieve a pathological complete response (51,52). Other publications have demonstrated a pathological complete response rate of 20–30% and this predicted for improved overall survival (53,54).
Since patients who achieved complete responses also achieved better long-term outcomes compared to those who did not, the question of whether this subset of patients could avoid or minimise surgery became an area of interest. Local excision after neoadjuvant therapy was investigated as an option of organ preservation.
GRECCAR 2 was a prospective, randomised, open-labelled, multicentre, phase 3 trial conducted at 15 tertiary centres in France with the aim of demonstrating superiority of local excision over TME for patients with T2-T3 lower rectal carcinoma, of maximum size 4 cm, who had a good clinical response to neoadjuvant chemoradiotherapy. Unfortunately, they failed to show superiority of local excision over TME, because many patients in the local excision group received a completion TME that likely increased morbidity and toxicity, compromising the potential advantages of local excision (55).
Non-operative approach
Habr-Gama et al. conducted a trial to investigate if a non-operative approach for stage 0 rectal cancer was feasible. Two-hundred and sixty-five patients with resectable distal rectal cancer received neoadjuvant long course chemo-RT. Patients were reassessed at 8 weeks following treatment, using clinical, radiological and endoscopic examinations. Seventy-one patients (26.8%) achieved a clinical complete response; they avoided immediate surgery and had to follow a strict surveillance program. After a median follow-up of 57.3 months, only two patients had a local recurrence, and three patients developed systemic disease. The 5-year overall survival and disease-free survival rates were 100% and 92%, respectively. This landmark study pioneered a daring new concept in rectal cancer management and championed the potential for organ preservation (56).
Maas et al. added further evidence to support this treatment strategy with his prospective cohort study using a “Wait and see” approach for clinical complete responders. They reported a 2-year disease-free survival and overall survival of 93% and 91%, respectively which was at least as good as that of patients with a pathological complete response after surgery (57).
A systematic review and meta-analysis of this approach found no significant difference between patients managed with watch-and-wait and patients with clinical complete response treated with surgery in terms of non-regrowth recurrence, cancer-specific mortality, disease-free survival or overall survival (58).
Table 2 summaries prospective studies investigating the non-operative approach (63).
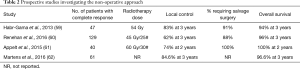
Full table
Total neoadjuvant therapy
In view of the promising results of this nonoperative approach to managing rectal cancer, total neoadjuvant therapy, incorporating both chemotherapy and concurrent chemoradiotherapy in the neoadjuvant setting is being investigated.
Cercek and colleagues reported the safety and efficacy of initial FOLFOX before chemo-RT and its effect on tumour downsizing and pathological complete response in patients with locally advanced rectal cancer. Twenty-two out of 61 patients (36%) had either pathological or clinical complete response. Forty-nine patients underwent surgery and all had R0 resections. Twenty-three (47%) had tumour response of more than 90%, including 13 (27%) with pathological complete response (64).
Markovina et al. reported improved metastasis- and disease-free survival with preoperative sequential short-course RT and FOLFOX chemotherapy when compared to neoadjuvant long course chemo-RT in a matched pair analysis. They compared treatment and toxicity outcomes between near total neoadjuvant therapy (nTNT) and the standard of care (neoadjuvant chemo-RT followed by surgery and adjuvant chemotherapy). Three-year disease-free survival (85% vs. 68%, P=0.032) was significantly better in the nTNT group. Three-year local control and overall survival were similar (65).
A multi-institutional, randomised phase II study is investigating the efficacy of total neoadjuvant therapy and selective non-operative management in rectal cancer patients. Patients with stage II or III rectal cancer will be randomized to receive FOLFOX/CAPEOX: (I) before induction neoadjuvant chemotherapy or (II) after consolidation neoadjuvant chemotherapy, with 5-FU or capecitabine-based chemoradiation. Those with residual tumour at the primary site will undergo surgery. Patients with clinical complete response will receive non-operative management (66).
As yet, there is no randomised phase 3 trial evidence to propel this treatment strategy into regular clinical practice but it does leave the future open to a potentially remarkable way of treating rectal cancer without surgery in selected cases.
Response assessment after RT
In a bid to better select the patients who would be suitable for the non-operative approach, investigators have studied various imaging modalities that would help to predict treatment response after neoadjuvant therapy.
Travaini et al. evaluated the ability of Positron emission tomography/computed tomography (PET/CT) with fluorodeoxyglucose (18F-FDG) to accurately predict treatment response after neoadjuvant chemo-RT. They found that responders (patients who achieved downstaging or downsizing) and non-responders had differences in their early and post-treatment maximal standardised uptake value (SUVmax) percent reduction (67).
In the MERCURY trial, magnetic resonance imaging (MRI)-assessed tumour regression grade and circumferential resection margin were imaging markers that could also predict better overall and disease-free survival for good responders to neoadjuvant therapy (68).
A meta-analysis and systematic review of using MRI found a mean sensitivity of 50% and specificity of 91% in detecting residual tumour using standard T2 weighted sequences during restaging MRI after neoadjuvant chemo-RT. Sensitivity was improved with diffusion-weighted imaging (DWI) or with experienced observers (69).
Ongoing trials
Thus far, all of treatment strategies have improved local control but few have an impact on overall survival. The RAPIDO trial aims to address this issue in the hope of improving overall survival for rectal cancer patients by delivering short-course RT (25 Gy/5#) followed by chemotherapy (capecitabine and oxaliplatin) in 6 cycles before surgery. This will be compared to the standard chemoradiation (45–50 Gy/25#with capecitabine) preoperatively, followed by selective postoperative adjuvant chemotherapy (70).
The PROSPECT trial is investigating preoperative chemotherapy alone compared to chemo-RT in a bid to decrease the toxicity of radiation for rectal cancer patients. The purpose of the trial is to compare the effects of the standard treatment of chemotherapy and radiation to chemotherapy alone with FOLFOX and selective use of the standard treatment, depending on response to the FOLFOX (71).
Biomarkers
Following in the footsteps of other organ subsites, we delve deeper into cancer genomics and investigate the ability of biomarkers to predict the responsiveness to neoadjuvant therapy.
Several biomarkers have been studied but few have declared themselves superior to others and few are in routine clinical use.
A meta-analysis investigating the ability of p53 status as a predictive biomarker for patients receiving neoadjuvant radiation-based treatment found that wild-type form of p53 status (low expression of p53 protein and/or wild-type p53 gene) was associated with pathologic response in these patients. The association between response and the presence of p53 gene mutations was stronger than that between response and protein positivity (72).
Circulating tumour cells (CTCs) were found to be advantageous over serum carcinoembryonic antigen in predicting treatment responses in rectal cancer. One hundred and three rectal cancer patients were analysed before and after chemoradiotherapy. CTCs were detected in all patients while none were detected in healthy controls. CTC levels in metastatic patients were significantly higher than those with localised disease. There was a close relationship between CTC levels and treatment outcomes unlike serum CEA which did not have any correlation (73).
D’Angelo et al. performed microRNA microarray analysis for 38 patients with locally advanced rectal cancer. He found 11 microRNAs that were significantly different between patients who were deemed responders (tumour regression grade 1–2) and non-responders (tumour regression grade 3–5). In particular, miR-125b serum levels were significantly overexpressed in non-responder patients compared to those who responded well (74).
RT in metastatic rectal cancer
Curative intent
A subset of patients with metastatic rectal cancer present with oligometastatic disease usually to the liver or lung.
A retrospective review compared patients with synchronous resectable liver or lung metastases who received neoadjuvant therapy (long course chemo-RT or short course RT) versus no neoadjuvant therapy, followed by resection of the primary tumour and metastasectomy. At a median follow up of 43 months, none of the patients who received RT had a local recurrence. The 5-year overall survival rates were: 43.3% for without RT vs. 58.3% with RT (75).
Leonard et al. reviewed their institutional experience involving patients with metastatic rectal cancer who were treated with RT to their primary tumour. Among the ten patients with oligometastatic disease, the local control rate was 90% and the 5-year overall survival rate was 70% compared to 30% for patients without oligometastatic disease (76).
The NCCN guidelines for patients with resectable oligometastatic rectal cancer offers the options of neoadjuvant short course RT or long course chemo-RT prior to surgery (38).
Palliative intent
RT is effective for advanced or metastatic rectal cancers when palliation of symptoms is required locally. A retrospective study of 99 patients demonstrated a response rate of 62.5% to 86.7% with median duration response from 4.2–5.4 months. The median survival was 6.9 months and there was no grade 4 toxicity (77).
A prospective, multicentre study showed similar results for locally advanced rectal cancer treated with palliative RT with a dose range of 30–39 Gy. Overall response rates were 85% and median survival was 9 months. There was no grade 4 toxicity (78).
Short course RT (25 Gy in 5 fractions) has also been investigated in the palliative setting. A phase 2 study enrolled 18 patients and found a complete response (complete symptom resolution) in 38.9% of patients, a partial response in 50.0% cases, and no response in 11.1%. Median survival was 25 months; 16.7% had grade 3 toxicity and there was no grade 4 toxicity (79).
Conclusions
The management of rectal cancer has evolved over time and as more research is conducted and published, we are continually striving towards improving patient outcomes and reducing treatment related toxicities for the benefit of the patients. Trimodality treatment remains necessary for high risk patients however total neoadjuvant therapy may improve the pathological complete response rates and allow for omission of surgery or the non-operative approach. However, it remains clear that we need innovative methods to risk stratify patients.
In time to come, what is currently accepted as standard of care may cease to be as we move into an era of personalised medicine and instead of subjecting all patients to standard treatment, we aim to tailor the treatment specifically for the patient or rather, their cancer, avoiding over-treatment or under-treatment.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Papillon J. Endocavity irradiation of early rectal cancers for cure: a series of 123 cases. Proc R Soc Med 1973;66:1179-81. [Crossref] [PubMed]
- Papillon J, Berard P. Endocavitary irradiation in the conservative treatment of adenocarcinoma of the low rectum. World J Surg 1992;16:451-7. [Crossref] [PubMed]
- Myint AS. Novel radiation techniques for rectal cancer. J Gastrointest Oncol 2014;5:212-7. [PubMed]
- Garcia-Aguilar J, Renfro LA, Chow OS, et al. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol 2015;16:1537-46. [Crossref] [PubMed]
- Althumairi AA, Gearhart SL. Local excision for early rectal cancer: transanal endoscopic microsurgery and beyond. J Gastrointest Oncol 2015;6:296-306. [PubMed]
- Greenberg JA, Shibata D, Herndon JE, et al. Local Excision of Distal Rectal Cancer: An Update of Cancer and Leukemia Group B 8984. Dis Colon Rectum 2008;51:1185-91; discussion 1191-4. [Crossref] [PubMed]
- Jeong JU, Nam TK, Kim HR, et al. Adjuvant chemoradiotherapy instead of revision radical resection after local excision for high-risk early rectal cancer. Radiat Oncol 2016;11:114. [Crossref] [PubMed]
- Lee S, Woo CG, Lee HJ, et al. Effectiveness of adjuvant radiotherapy after local excision of rectal cancer with deep submucosal invasion: a single-hospital, case-control analysis. Surg Endosc 2015;29:3231-8. [Crossref] [PubMed]
- Sun G, Tang Y, Li X, et al. Analysis of 116 cases of rectal cancer treated by transanal local excision. World J Surg Oncol 2014;12:202. [Crossref] [PubMed]
- Suzuki T, Sadahiro S, Tanaka A, et al. Outcomes of Local Excision plus Chemoradiotherapy in Patients with T1 Rectal Cancer. Oncology 2018;95:246-50. [Crossref] [PubMed]
- Russell AH, Harris J, Rosenberg PJ, et al. Anal sphincter conservation for patients with adenocarcinoma of the distal rectum: long-term results of radiation therapy oncology group protocol 89-02. Int J Radiat Oncol Biol Phys 2000;46:313-22. [Crossref] [PubMed]
- Steele GD Jr, Herndon JE, Bleday R, et al. Sphincter-sparing treatment for distal rectal adenocarcinoma. Ann Surg Oncol 1999;6:433-41. [Crossref] [PubMed]
- Mendenhall WM, Morris CG, Rout WR, et al. Local excision and postoperative radiation therapy for rectal adenocarcinoma. Int J Cancer 2001;96 Suppl:89-96. [Crossref] [PubMed]
- Heald RJ, Moran BJ, Ryall RD, et al. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg 1998;133:894-9. [Crossref] [PubMed]
- Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet 2001;358:1291-304. [Crossref] [PubMed]
- Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst 1988;80:21-9. [Crossref] [PubMed]
- Gastrointestinal Tumor Study Group. Prolongation of the Disease-Free Interval in Surgically Treated Rectal Carcinoma. N Engl J Med 1985;312:1465-72. [Crossref] [PubMed]
- Krook JE, Moertel CG, Gunderson LL, et al. Effective Surgical Adjuvant Therapy for High-Risk Rectal Carcinoma. N Engl J Med 1991;324:709-15. [Crossref] [PubMed]
- O'Connell MJ, Martenson JA, Wieand HS, et al. Improving Adjuvant Therapy for Rectal Cancer by Combining Protracted-Infusion Fluorouracil with Radiation Therapy after Curative Surgery. N Engl J Med 1994;331:502-7. [Crossref] [PubMed]
- Wolmark N, Wieand HS, Hyams DM, et al. Randomized Trial of Postoperative Adjuvant Chemotherapy With or Without Radiotherapy for Carcinoma of the Rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst 2000;92:388-96. [Crossref] [PubMed]
- NIH consensus conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA 1990;264:1444-50. [Crossref] [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus Postoperative Chemoradiotherapy for Rectal Cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33. [Crossref] [PubMed]
- Roh MS, Colangelo LH, O'Connell MJ, et al. Preoperative Multimodality Therapy Improves Disease-Free Survival in Patients With Carcinoma of the Rectum: NSABP R-03. J Clin Oncol 2009;27:5124-30. [Crossref] [PubMed]
- Swedish Rectal Cancer Trial, Cedermark B, Dahlberg M, et al. Improved Survival with Preoperative Radiotherapy in Resectable Rectal Cancer. N Engl J Med 1997;336:980-7. [Crossref] [PubMed]
- Folkesson J, Birgisson H, Pahlman L, et al. Swedish Rectal Cancer Trial: Long Lasting Benefits From Radiotherapy on Survival and Local Recurrence Rate. J Clin Oncol 2005;23:5644-50. [Crossref] [PubMed]
- Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46. [Crossref] [PubMed]
- Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet 2009;373:811-20. [Crossref] [PubMed]
- Kye BH, Cho HM. Overview of radiation therapy for treating rectal cancer. Ann Coloproctol 2014;30:165-74. [Crossref] [PubMed]
- Bosset JF, Collette L, Calais G, et al. Chemotherapy with Preoperative Radiotherapy in Rectal Cancer. N Engl J Med 2006;355:1114-23. [Crossref] [PubMed]
- Wagman R, Minsky BD, Cohen AM, et al. Sphincter preservation in rectal cancer with preoperative radiation therapy and coloanal anastomosis: long term follow-up. Int J Radiat Oncol Biol Phys 1998;42:51-7. [Crossref] [PubMed]
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006;93:1215-23. [Crossref] [PubMed]
- Ngan SY, Burmeister B, Fisher RJ, et al. Randomized Trial of Short-Course Radiotherapy Versus Long-Course Chemoradiation Comparing Rates of Local Recurrence in Patients With T3 Rectal Cancer: Trans-Tasman Radiation Oncology Group Trial 01.04. J Clin Oncol 2012;30:3827-33. [Crossref] [PubMed]
- Guerrero Urbano MT, Henrys AJ, Adams EJ, et al. Intensity-modulated radiotherapy in patients with locally advanced rectal cancer reduces volume of bowel treated to high dose levels. Int J Radiat Oncol Biol Phys 2006;65:907-16. [Crossref] [PubMed]
- David J, Jabbour S, Gresham GK, et al. Effect of neoadjuvant IMRT for locally advanced rectal cancer on toxicity and pathologic response. J Clin Oncol 2017;35:693. [Crossref]
- Tey J, Leong CN, Cheong WK, et al. A phase II trial of preoperative concurrent chemotherapy and dose escalated intensity modulated radiotherapy (IMRT) for locally advanced rectal cancer. J Cancer 2017;8:3114-21. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Rectal Cancer (Version 3.2018). Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf
- Hesselager C, Vuong T, Påhlman L, et al. Short-term outcome after neoadjuvant high-dose-rate endorectal brachytherapy or short-course external beam radiotherapy in resectable rectal cancer. Colorectal Dis 2013;15:662-6. [Crossref] [PubMed]
- Buckley H, Wilson C, Ajithkumar T. High-Dose-Rate Brachytherapy in the Management of Operable Rectal Cancer: A Systematic Review. Int J Radiat Oncol Biol Phys 2017;99:111-27. [Crossref] [PubMed]
- Rijkmans EC, Cats A, Nout RA, et al. Endorectal Brachytherapy Boost After External Beam Radiation Therapy in Elderly or Medically Inoperable Patients With Rectal Cancer: Primary Outcomes of the Phase 1 HERBERT Study. Int J Radiat Oncol Biol Phys 2017;98:908-17. [Crossref] [PubMed]
- Erickson BA, Demanes DJ, Ibbott GS, et al. American Society for Radiation Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys 2011;79:641-9. [Crossref] [PubMed]
- Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the Interval Between Preoperative Radiation Therapy and Surgery on Downstaging and on the Rate of Sphincter-Sparing Surgery for Rectal Cancer: The Lyon R90-01 Randomized Trial. J Clin Oncol 1999;17:2396. [Crossref] [PubMed]
- Habr-Gama A, Perez RO, Proscurshim I, et al. Interval Between Surgery and Neoadjuvant Chemoradiation Therapy for Distal Rectal Cancer: Does Delayed Surgery Have an Impact on Outcome? Int J Radiat Oncol Biol Phys 2008;71:1181-8. [Crossref] [PubMed]
- Macchia G, Gambacorta MA, Masciocchi C, et al. Time to surgery and pathologic complete response after neoadjuvant chemoradiation in rectal cancer: A population study on 2094 patients. Clin Transl Radiat Oncol 2017;4:8-14. [Crossref] [PubMed]
- Lefevre JH, Mineur L, Kotti S, et al. Effect of Interval (7 or 11 weeks) Between Neoadjuvant Radiochemotherapy and Surgery on Complete Pathologic Response in Rectal Cancer: A Multicenter, Randomized, Controlled Trial (GRECCAR-6). J Clin Oncol 2016;34:3773-80. [Crossref] [PubMed]
- Tulchinsky H, Shmueli E, Figer A, et al. An Interval >7 Weeks between Neoadjuvant Therapy and Surgery Improves Pathologic Complete Response and Disease–Free Survival in Patients with Locally Advanced Rectal Cancer. Ann Surg Oncol 2008;15:2661-7. [Crossref] [PubMed]
- Moore HG, Gittleman AE, Minsky BD, et al. Rate of Pathologic Complete Response With Increased Interval Between Preoperative Combined Modality Therapy and Rectal Cancer Resection. Dis Colon Rectum 2004;47:279-86. [Crossref] [PubMed]
- Sloothaak DA, Geijsen DE, van Leersum NJ, et al. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 2013;100:933-9. [Crossref] [PubMed]
- Erlandsson J, Holm T, Pettersson D, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol 2017;18:336-46. [Crossref] [PubMed]
- Leong YH, Leong CN, Tay GS, et al. Oncologic outcomes of neoadjuvant chemoradiation for locally advanced rectal cancer: a single-institution experience. Ann Acad Med Singapore 2014;43:569-75. [PubMed]
- Tseng MSF, Zheng H, Ng IWS, et al. Outcomes of neoadjuvant chemoradiotherapy followed by total mesorectal excision surgery for locally advanced rectal cancer: a single-institution experience. Singapore Med J 2018;59:305-10. [Crossref] [PubMed]
- Janjan NA, Crane C, Feig BW, et al. Improved overall survival among responders to preoperative chemoradiation for locally advanced rectal cancer. Am J Clin Oncol 2001;24:107-12. [Crossref] [PubMed]
- Fokas E, Liersch T, Fietkau R, et al. Tumor Regression Grading After Preoperative Chemoradiotherapy for Locally Advanced Rectal Carcinoma Revisited: Updated Results of the CAO/ARO/AIO-94 Trial. J Clin Oncol 2014;32:1554-62. [Crossref] [PubMed]
- Rullier E, Rouanet P, Tuech JJ, et al. Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open-label, multicentre, phase 3 trial. Lancet 2017;390:469-79. [Crossref] [PubMed]
- Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 2004;240:711-7; discussion 717-8. [PubMed]
- Maas M, Beets-Tan RGH, Lambregts DMJ, et al. Wait-and-See Policy for Clinical Complete Responders After Chemoradiation for Rectal Cancer. J Clin Oncol 2011;29:4633-40. [Crossref] [PubMed]
- Dossa F, Chesney TR, Acuna SA, et al. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017;2:501-13. [Crossref] [PubMed]
- Habr-Gama A, Sabbaga J, Gama-Rodrigues J, et al. Watch and Wait Approach Following Extended Neoadjuvant Chemoradiation for Distal Rectal Cancer. Dis Colon Rectum 2013;56:1109-17. [Crossref] [PubMed]
- Renehan AG, Malcomson L, Emsley R, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol 2016;17:174-83. [Crossref] [PubMed]
- Appelt AL, Pløen J, Harling H, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol 2015;16:919-27. [Crossref] [PubMed]
- Martens MH, Maas M, Heijnen LA, et al. Long-term Outcome of an Organ Preservation Program After Neoadjuvant Treatment for Rectal Cancer. J Natl Cancer Inst 2016. [Crossref] [PubMed]
- Qian Y, Chin AL, Toesca DAS, et al. Nonoperative Management of Rectal Cancer: A Modern Perspective. Oncology (Williston Park) 2017;31:e13-22. [PubMed]
- Cercek A, Goodman KA, Hajj C, et al. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Canc Netw 2014;12:513-9. [Crossref] [PubMed]
- Markovina S, Youssef F, Roy A, et al. Improved Metastasis- and Disease-Free Survival With Preoperative Sequential Short-Course Radiation Therapy and FOLFOX Chemotherapy for Rectal Cancer Compared With Neoadjuvant Long-Course Chemoradiotherapy: Results of a Matched Pair Analysis. Int J Radiat Oncol Biol Phys 2017;99:417-26. [Crossref] [PubMed]
- Smith JJ, Chow OS, Gollub MJ, et al. Organ Preservation in Rectal Adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC cancer 2015;15:767. [Crossref] [PubMed]
- Travaini LL, Zampino MG, Colandrea M, et al. PET/CT with Fluorodeoxyglucose During Neoadjuvant Chemoradiotherapy in Locally Advanced Rectal Cancer. Ecancermedicalscience 2016;10:629. [Crossref] [PubMed]
- Patel UB, Taylor F, Blomqvist L, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 2011;29:3753-60. [Crossref] [PubMed]
- van der Paardt MP, Zagers MB, Beets-Tan RGH, et al. Patients Who Undergo Preoperative Chemoradiotherapy for Locally Advanced Rectal Cancer Restaged by Using Diagnostic MR Imaging: A Systematic Review and Meta-Analysis. Radiology 2013;269:101-12. [Crossref] [PubMed]
- Nilsson PJ, van Etten B, Hospers GAP, et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer – the RAPIDO trial. BMC Cancer 2013;13:279. [Crossref] [PubMed]
- PROSPECT: Chemotherapy Alone or Chemotherapy Plus Radiation Therapy in Treating Patients With Locally Advanced Rectal Cancer Undergoing Surgery. ClinicalTrials.gov Identifier: NCT0151578.
- Chen MB, Wu XY, Yu R, et al. P53 status as a predictive biomarker for patients receiving neoadjuvant radiation-based treatment: a meta-analysis in rectal cancer. PLoS One 2012;7:e45388. [Crossref] [PubMed]
- Sun W, Huang T, Li G, et al. The advantage of circulating tumor cells over serum carcinoembryonic antigen for predicting treatment responses in rectal cancer. Future Oncol 2013;9:1489-500. [Crossref] [PubMed]
- D’Angelo E, Fassan M, Maretto I, et al. Serum miR-125b is a non-invasive predictive biomarker of the pre-operative chemoradiotherapy responsiveness in patients with rectal adenocarcinoma. Oncotarget 2016;7:28647-57. [PubMed]
- Fossum CC, Alabbad JY, Romak LB, et al. The role of neoadjuvant radiotherapy for locally-advanced rectal cancer with resectable synchronous metastasis. J Gastrointest Oncol 2017;8:650-8. [Crossref] [PubMed]
- Leonard KL, Rava PS, DiPetrillo TA. The Role of Local Radiation Therapy in the Treatment of Metastatic Rectal Cancer. Int J Radiat Oncol Biol Phys 2012;84:S347. [Crossref]
- Chia D, Lu J, Zheng H, et al. Efficacy of palliative radiation therapy for symptomatic rectal cancer. Radiother Oncol 2016;121:258-61. [Crossref] [PubMed]
- Cameron MG, Kersten C, Vistad I, et al. Palliative pelvic radiotherapy for symptomatic rectal cancer – a prospective multicenter study. Acta Oncologica 2016;55:1400-7. [Crossref] [PubMed]
- Picardi V, Deodato F, Guido A, et al. Palliative Short-Course Radiation Therapy in Rectal Cancer: A Phase 2 Study. Int J Radiat Oncol Biol Phys 2016;95:1184-90. [Crossref] [PubMed]


