Adjuvant chemoradiation for pancreatic cancer: what does the evidence tell us?
Background
Minimal progress has been made to significantly improve treatment outcomes for pancreas cancer patients despite constant efforts to better understand this devastating disease. According to the American Cancer Society, the 5-year overall survival (OS) rate has only marginally increased from 2% between 1975-1977 to 6% between 2003-2009 (1). The roadblocks to major progress are predominantly related to limitations in early cancer diagnosis when tumors are more likely to be resectable as well as poor detection of occult locoregional and distant metastasis.
While the goal for pancreas cancer patients is ultimately to achieve a margin negative (R0) resection, this is not possible for the majority of newly diagnosed patients typically either due to distant metastatic spread or extensive locoregional involvement of critical vascular structures. The minority of newly diagnosed patients who successfully undergo a R0 resection are at an extremely high risk for both locoregional and distant disease recurrence (2-8). Therefore, adjuvant therapy is the standard of care for resected pancreas cancer. While the benefit of adjuvant chemotherapy is undisputed, the addition of radiation therapy (RT) remains hugely controversial (6,9,10). In this article we will review the published literature with respect to adjuvant RT and optimal patient selection, treatment techniques, and incorporation of systemic agents.
Historical randomized trials
The initial studies that evaluated the addition of postoperative chemoradiation (CRT) for pancreas cancer are extensively discussed and debated in the published literature. Fueling this debate is the combination of limited prospective randomized data comparing the use of adjuvant CRT to no adjuvant CRT, conclusions made by older trials that used outdated RT techniques, and numerous flaws in the design and execution of these historical trials. That being said, we should critically interpret these trials when making treatment recommendations to our patients and when designing future trials that further examine how best to implement adjuvant CRT.
The Gastrointestinal Tumor Study Group (GITSG) 9173 trial was the first to evaluate whether surgery followed by adjuvant CRT would improve outcomes over surgery alone for resected pancreas patients (11). This trial of 43 patients limited enrollment to only those with negative surgical margins. The authors reported a significant OS benefit favoring the CRT arm despite the trial closing early due to poor accrual. In contrast to how we would treat these patients today, RT was delivered to 40 Gy in 20 fractions with a planned 2-week break after 20 Gy. 5-fluorouracil (5-FU) was given concurrently and after RT for 2 years or until evidence of disease progression. After an additional 30 patients were treated on a non-randomized arm using the same CRT regimen and had similar survival as those from the randomized CRT arm, CRT was considered to be a new standard of care for resected pancreas cancer management (12).
Several European studies were subsequently conducted that challenged whether CRT actually improved survival. The European Organization for Research and Treatment of Cancer (EORTC) randomized patients to surgery alone versus surgery followed by CRT, as was done in the GITSG trial (13). The EORTC trial did not demonstrate a significant survival benefit favoring CRT, although a trend towards improved survival emerged for the subset of patients with pancreatic head tumors (13,14). While many interpret this as a negative trial, others have countered that a number of flaws in trial execution and design likely prevented any CRT benefit from being detected. First, whereas the GITSG only included pancreas cancers nearly 50% of the patients enrolled on the EORTC trial had periampullary tumors, which have a more favorable prognosis. Second, 20% of patients did not receive adjuvant therapy despite being randomized to receive CRT and 44% did not receive chemotherapy per protocol. Third, the EORTC enrolled patients with positive surgical margins without stratifying by margin status while the GITSG excluded patients with positive margins. Fourth, while patients on the GITSG trial received maintenance chemotherapy, this was not given in the EORTC trial. Lastly, some have argued that if the EORTC data were evaluated using a one-sided instead of a two-sided log rank test, then this would have provided statistical significance (P=0.049) to the survival improvement seen with adjuvant CRT (15). Still, Europeans cite this as a negative trial and typically recommend adjuvant chemotherapy alone.
The European Study for Pancreatic Cancer (ESPAC)-1 trial concluded that not only was there no survival benefit obtained by using adjuvant CRT, but also that CRT actually caused a detriment in survival (16). This is the largest prospective study to evaluate adjuvant therapy for pancreas cancer patients, randomizing 254 patients from 61 European institutions after surgery either to chemotherapy alone versus observation or CRT versus observation. An additional 285 patients were included in a 2×2 factorial randomization between observation, chemotherapy alone, CRT alone, and CRT followed by maintenance chemotherapy. In a 2004 report of the patients treated within the 2×2 factorial design, CRT negatively affected 5-year OS versus no CRT (10% vs. 20%; P=0.05) while chemotherapy improved 5-year OS compared to no chemotherapy (21% vs. 8%; P=0.009). This trial has been widely criticized due to the ability of the treating physician to choose the randomization, the use of “background” therapy, lack of central review, and longer time to treatment in the CRT arm (17,18). Several more recent studies have specifically refuted the claim that CRT is detrimental to survival (19,20). Kinsella et al. examined whether unfavorable results in the CRT arm from the ESPAC-1 trial could be related to inadequate radiation delivery (20). They matched pT3N1 patients from the ESPAC-1 trial who were treated per their institutional regimen of 63 Gy and concurrent chemotherapy and concluded that the observed survival outcomes from the ESPAC-1 trial were dramatically inferior to those that would be “expected” using modern and high quality CRT. In fact, the observed results were outside the 95% confidence intervals for “expected” survival. While speculative, these data emphasize that CRT was not fairly assessed in the ESPAC-1 trial.
The next phase III study to include adjuvant CRT was Radiation Therapy Oncology Group (RTOG) 9704, which randomized patients to 5-FU CRT sandwiched between either gemcitabine or 5-FU (21). After an initial report with a median follow up of 4 years showing a significant improvement in survival, with additional follow up (median =7 years), only a trend towards improved survival was detected for pancreatic head tumors treated with gemcitabine (median survival 20.5 vs. 17.1 months; P=0.08) (22). This was felt to be potentially related to the interruption of gemcitabine via the “sandwiched” 5-FU CRT and hence became a consideration in the design of the successor trial.
RTOG 0848, the successor study to RTOG 9704, is a phase III trial that is attempting to answer two questions, the first being whether there is a survival benefit for adding erlotinib to gemcitabine compared to gemcitabine alone among head of pancreas patients who have undergone either an R0 or R1 resection. The second question is whether the addition of CRT in patients who have no evidence of disease progression following a full course of gemcitabine is superior to full course of gemcitabine alone. The results of RTOG 0848 will be critical to shedding light on the role of CRT, and until they are available we have no choice but to look to published literature from the modern era to guide our clinical practice.
Recent studies using modern RT doses and delivery techniques do not universally agree that adjuvant CRT should be used over chemotherapy alone. For instance, results from a randomized phase II trial published in 2010 did not show a difference in survival among resected patients who received CRT in addition to gemcitabine versus gemcitabine alone, although the authors acknowledge that the trial was not designed to detect such a difference (23). Another recently published single institution study of 146 patients actually reported higher median survival in patients who received chemotherapy alone compared to CRT (21.5 versus 16.8 months), although this difference was not statistically significant (P=0.76). On the other hand, recent studies that perhaps most strongly advocate for the use of CRT are from the Mayo Clinic and Johns Hopkins University (19,24-26). A large collaborative study between these two high volume pancreas institutions included 1,386 resected patients (19). When compared to surgery alone, adjuvant CRT improved survival in propensity score analysis by 33% (P<0.001). Matched-pair analyses demonstrated prolonged median survival with CRT (21.9 vs. 14.3 months; P<0.001). The survival benefit favoring CRT over surgery alone was also reported individually by each institution (24-26). Interestingly, the median survival of 21.2 months reported in patients who received CRT at Johns Hopkins was remarkably similar to what was reported in the CRT arm of the GITSG trial (20 months) despite the Johns Hopkins patients having more high-risk features such as positive lymph nodes (80% vs. 30%) and positive surgical margins (45% vs. 0%). While a direct comparison cannot be made between these two studies, modern high quality RT likely improves outcomes compared to the poorly delivered RT used in the previously mentioned historical trials. This observation was demonstrated in RTOG 9704, the first phase III trial which required central quality assurance review of RT fields used (27).
Personalized therapy
Despite adjuvant CRT not being universally adopted, it is generally agreed that a subset of resected patients with a high risk for locoregional disease recurrence may particularly benefit from the addition of RT to chemotherapy (28). For example, RT did not seem to benefit patients in the aforementioned Mayo Clinic experience who did not have any specified negative risk factors while those with at least one negative risk factor did have significantly improved survival (24). Other studies support this strategy in patients with negative features such as older age, large tumor size, advanced tumor stage, high histologic grade, elevated CA 19-9 level, positive lymph nodes, and positive surgical margins (4,24,26,29-34). The literature supports pathologic lymph node status, surgical margin status, and CA 19-9 level as being among the most important.
Lymph node involvement is consistently described as one of the most significant negative prognostic factors for long-term survival after surgery for pancreas cancer (21,30-33,35-37). Merchant and colleagues published a review of 747 pancreas patients from across seven academic medical institutions who had either surgery alone (n=374) or surgery followed by CRT (n=299) (35). While median OS was longer in patients receiving CRT (20 vs. 14.5 months, P=0.001), subset analysis showed that only the node positive patients benefitted (HR 0.477; 95% CI, 0.357-0.638, P<0.0001). The survival benefit of CRT has repeatedly been demonstrated among node positive patients using the Surveillance, Epidemiology, and End Results (SEER) database, although Mellon et al. were the first to demonstrate that RT conferred a survival benefit despite including information on chemotherapy in their analysis (31,38-41). The importance of lymph node metastasis was also shown in the analysis of RTOG 9704 (21). The data from RTOG 9704 were further analyzed by Showalter et al. to better understand the importance of certain lymph node parameters beyond only classifying patients as either having or not having lymph node metastasis (32). Their conclusions were in agreement with work previously published by others that showed a significant association between worse OS and higher number of positive nodes (NPN) (33,42,43), fewer total nodes examined (TNE) (31,43-45), and higher lymph node ratio (LNR) (45-48). While there is substantial evidence that lymph node involvement portends worse outcomes, we should be aware that node positive disease does not necessarily preclude long-term survival as shown in a study by Schnelldorfer et al. in which 32% of the 62 patients alive at 5 years and 29% of the 21 patients alive at 10 years had pathologically positive nodes (37).
Surgical margin status has also been described as being a highly significant negative prognostic factor. Patients who undergo resection with negative surgical margins (R0) have prolonged survival over those who have either microscopically positive (R1) or grossly positive (R2) margins (20,26,33-35,49-51). However, some investigators question the significance of postoperative margin status (52-54). A Pancreatic Cancer Meta-analysis Group (PCMG) study suggested that resection margin status was not a significant factor for survival, although R1 patients had a 28% reduction in the risk of death after CRT (52). Perhaps the benefit of R0 resection is not uniform, as suggested by Tummala et al., who showed a dramatic improvement in survival for R0 versus R1 resection, but only for patients with tumors no larger than 25 mm who also had no more than one positive lymph node (55).
The importance of postoperative CA 19-9 levels was most prominently demonstrated by RTOG 9704 in which a secondary endpoint was survival based on a postoperative CA 19-9 cutoff of 180 U/mL. The 5-year survival of patients with CA 19-9 ≥180 U/mL was 0% compared to 25% and 18% in patients with CA 19-9 <180 U/mL treated with either gemcitabine or 5-FU, respectively. In addition, the authors analyzed the RTOG 9704 data using a threshold of 90 U/mL, inspired by the CONKO-001 trial that only included patients with values <90 U/mL. As was seen using the higher cutoff, patients with CA 19-9 <90 U/mL also had significantly higher 5-year OS (23% vs. 2%; P<0.0001). Finally, the most important independent predictor of survival in multivariate analyses from RTOG 9704 was postresection CA 19-9 using the cutoffs of 90 U/mL [HR 3.02; P<0.0001 (95% CI, 2.16-4.23)] and 180 U/mL [HR 3.18; P<0.0001 (95% CI, 2.09-4.84)]. Preoperative CA 19-9 level is also thought to be a useful prognostic factor as supported by multiple single institution retrospective reports (56-59), the largest of which was published by the Mayo Clinic (56). Of 226 patients, approximately half received adjuvant CRT alone (n=122) with the remainder receiving CRT followed by additional chemotherapy (n=23), chemotherapy alone (n=6), or observation (n=69). Adjuvant CRT was delivered to a median 50.4 Gy and nearly all received concurrent infusional 5-FU. Multivariate analysis showed preoperative CA 19-9 levels based on cutoffs of 180 and 90 U/mL to each significantly predict survival. Survival was significantly higher among the 101 patients with preoperative CA 19-9 ≥180 U/mL who received adjuvant CRT compared to those who did not (median survival 16.8 vs. 11.4 months; 5-year OS 24% vs. 5%; P<0.001). Lastly, the utility of preoperative CA 19-9 level may also include the ability to predict for tumor stage, nodal involvement, tumor grade, and surgical margin status. Prospective studies are needed to clarify the importance of preoperative CA 19-9, preoperative versus postoperative CA 19-9, and the ideal CA 19-9 cutoff.
There is increasing awareness that certain biomarkers may correlate with survival (60-62). Arguably the most promising of these is the tumor suppression gene DPC4 (SMAD4), which encodes the Smad4 protein involved in the transforming growth factor (TGF)-β signaling pathway. Smad4 status appears to be associated with patterns of failure; intact Smad4 patients seem to predominantly recur locally while those with loss of Smad4 are more likely to have distant progression (63-65). Herman et al. recently evaluated Smad4 status in 29 resected pancreas patients and discovered that recurrence-free survival was prolonged in patients with intact Smad4 (17.4 vs. 11.5 months; P=0.003), although there was no OS difference based on Smad4 status (64).
At this time, it is not clear how to precisely incorporate certain prognostic factors within our clinical practice. However, adjuvant CRT should be strongly considered in patients with multiple high-risk features such as positive lymph nodes and positive margins. If Smad4 status proves to reliably predict patterns of recurrence, then patients with intact Smad4 may particularly benefit from adjuvant CRT.
Evolution of radiation therapy (RT) techniques
We should be mindful that interpretation of study results should be within the context of the treatment era and the specific treatment delivered. The previously mentioned historical CRT trials used what is now undoubtedly considered to be antiquated RT including 2-dimensional planning and split-course radiation to a low dose.
In the decades that have followed these initial trials, technological advancements have included 3-dimensional conformal RT (3DCRT) and more recently intensity modulated radiation therapy (IMRT). IMRT is increasingly being used for pancreas cancer as well as other upper abdominal malignancies based on its superior ability to deliver sharp dose gradients at the periphery of the target volume, thereby significantly limiting unintended high dose to nearby normal tissues (Figure 1) (66-69). Even further normal tissue sparing may also be achieved using IMRT with noncoplanar beam angles (70), helical tomotherapy (69,71), and dose painting (72). Yovino et al. published the first comprehensive report of adjuvant IMRT in 71 pancreas cancer patients (34). They reported a low rate of locoregional failure (19%), alleviating concerns that the high conformality of IMRT did not lead to a compromise in treatment accuracy compared to less conformal techniques such as 3DCRT. In addition, treatment was very well tolerated with a much lower incidence of severe acute and late GI toxicity than would be expected using 3DCRT (34). Because of these favorable outcomes, both 3DCRT and IMRT may be used in RTOG 0848.
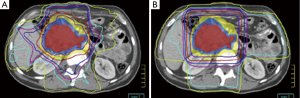
Although IMRT plans delivered using photons are incredibly conformal, the physical properties of protons allow for even greater sparing of normal tissues and delivery of lower integral dose. While dose in a photon beam decreases exponentially with increasing tissue depth, dose in a photon beam remains relatively constant until it reaches an area of maximal energy deposition, also known the Bragg peak. Thus, the main advantage of proton beam therapy (PBT) is that there is almost no dose delivered beyond the Bragg peak. While clinical PBT data is lacking for resected pancreas patients, there is data to suggest that PBT offers a dosimetric advantage over highly conformal photon therapy. Investigators at the University of Florida and University of Maryland generated PBT plans using simulation CT scans of eight resected patients who received IMRT. Each PBT plan was generated without knowledge of the corresponding IMRT plan dose distributions. The study authors demonstrated that the PBT and IMRT plans resulted in equivalent target coverage, although PBT was able to better limit dose to normal organs. PBT reduced median small bowel V20 from 47% to 15%, median gastric V20 from 20% to 2%, and median right kidney V18 from 51% to 27%. The University of Florida is now conducting a phase II trial (NCT01553019) of adjuvant CRT using PBT and concurrent chemotherapy.
Finally, there is increasing evidence that stereotactic body radiation therapy (SBRT) may benefit some patients with pancreas cancer although data in the postoperative setting is limited. SBRT is a technique that allows for large ablative doses to be precisely delivered to small focal targets in up to five fractions. Such large doses are thought to have a unique biologic effect and result in an enhanced local effect over standard fraction doses (73). While the pancreas SBRT literature focuses primarily on locally advanced disease (74,75), there is increasing enthusiasm to evaluate its use in borderline resectable (76) and even resectable patients (77). Rwigema et al. published a retrospective review of 24 resected pancreas patients who received SBRT, most commonly in a single fraction, for close or positive margins. No grade 3 or higher toxicities were noted while freedom from local progression was 95% at 6 months and 66% at 1 year. The utility of SBRT in the adjuvant setting remains to be seen.
Radiation dose and delivery schedule
Split-course RT, which was used in the GITSG, EORTC, and ESPAC-1 trials, prolongs overall treatment time and results in inferior local control due to accelerated repopulation (30,78). The use of a split-course approach to a lower dose than what is used today (40 Gy) was necessitated by the lack of highly conformal RT delivery resulting in significant dose to large amounts of normal organs. However, modern delivery techniques such as 3DCRT have allowed for doses of at least 50 Gy to be evaluated in prospective trials such as RTOG 9704 (21,79-81). Although we have the ability to safely deliver doses above 50 Gy, does it mean we should routinely do so? Few studies have measured the impact of RT dose on clinical outcomes for pancreas cancer (82,83). While dose escalation may benefit patients with gross disease (84), it remains unclear whether this holds true for patients with microscopic disease in the postoperative setting. Hall and colleagues recently examined the relationship between RT dose and survival in a cohort of 1,385 non-metastatic resected pancreas cancer patients (82). Most had positive lymph nodes (61.7%) and negative margins (71.3%). Median survival was longest in patients who received 50 to <55 Gy (n=498; 23 months) compared to those who received ≥55 Gy (n=89; 16 months), 40-50 Gy (n=634; 20 months), or <40 Gy (n=164; 15 months). Multivariate analysis revealed that in comparison to the reference range of 50 to <55 Gy, worse OS was predicted by <40 Gy [HR 1.30; (95% CI, 1.03-1.66); P=0.031], 40 to <50 Gy [HR 1.17; (95% CI, 1.00-1.37); P=0.05], and ≥55 Gy [HR 1.44; (95% CI, 1.08-1.93); P=0.013]. There was no significant difference between each group with respect to age, surgical margin status, nodal involvement, tumor size, or tumor stage.
Therefore, modern studies using highly conformal RT delivery and doses of approximately 50 Gy may better reflect the benefit of adjuvant CRT compared to older studies that used split-course RT to 40 Gy (24-26). Furthermore, these older studies did not require central quality assurance of RT plans, which we have learned is critical and can significantly affect OS (27).
What is the appropriate clinical target volume (CTV)?
The predilection of pancreas cancer to involve locoregional lymph nodes has long been recognized, with rates reported from clinical and pathological series of up to 80% (2-8). Imaging studies including CT, PET/CT, and MRI are not able to readily detect subclinical disease (6,85). Therefore, given the high likelihood of subclinical nodal involvement, many radiation oncologists agree that elective nodal irradiation (ENI) should be a standard component of treatment field design for both resectable and borderline resectable pancreas cancer. However, there is not a consensus regarding the use of ENI. Many have argued for omitting ENI altogether (86), particularly in the setting of locally advanced pancreas cancer, especially given the increasing use of SBRT (74,76). Others have favored extensive surgical lymph node interrogation of even the para-aortic nodes (87) despite data suggesting that this may not result in a survival benefit (88).
For the majority of radiation oncologists who utilize ENI, the required extent of lymph node coverage has been somewhat uncertain although this recently has become better characterized (89-92). Brunner et al. were the first to publish evidence-based guidelines for target volume delineation in resected head of pancreas patients. These were based on a histopathologic analysis of 178 patients who also had a formal regional systematic lymph node dissection (89). They described a systematic stepwise method by which radiation target volumes should be constructed based on factors including the frequency of nodal spread, respiratory motion, and expected treatment-related toxicity related to treatment volume. In accordance with previously published data, the peripancreatic and pancreaticoduodenal nodes were most commonly involved (93). The authors highlighted the importance of also including the celiac axis, para-aortic, superior mesenteric artery, and hepatoduodenal ligament regions based on their frequency of subclinical involvement. While coverage of these regions would significantly increase the treatment volume, the authors’ opinion was that the likelihood of tumor recurrence was outweighed by a potential increase in normal tissue injury. These data has served as the foundation for CTVs that are currently used today.
Sun et al. performed an extensive review of the published literature to comprehensively evaluate lymph node positivity rates and patterns of nodal spread in both resected head and body/tail pancreatic cancer patients (91). They included 18 studies representing 5,954 patients that provided a detailed lymph node analysis, including the paper by Brunner and colleagues. They concluded that the pattern and frequency of subclinical nodal involvement was consistent across all of the included studies. Caravatta et al. developed guidelines for CTV delineation based on these data published by Sun and colleagues (92). Lymph node regions with at least 3% risk of involvement were considered to be at a clinically significant risk of recurrence, and therefore were included in the CTV. The authors justified this 3% threshold as being appropriate because if the more commonly threshold of 10-15% was used, several classically included nodal groups such as the celiac axis and hepatoduodenal ligament would be excluded. They admit that their proposed target volumes for head of pancreas cancers were actually “quite comparable” to those as described by Brunner and colleagues.
The RTOG has published target volume delineation guidelines in an attempt to standardize target volume delineation for patients treated on RTOG 0848, given the importance of delivering high quality RT (27,94). These guidelines are in large part based on the previously reviewed data that described patterns of spread. The authors admit that the appropriate CTV definition after a pancreaticoduodenectomy remains uncertain, and that the results of RTOG 0848 will hopefully clarify this.
Finally, some have challenged whether smaller target volumes may effectively allow for dose escalation and decreased treatment toxicity without compromising local control (90,95). To guide target volume construction, investigators from Johns Hopkins University first mapped local recurrences with respect to easily identifiable and reproducible vascular structures including the celiac axis, SMA, and renal veins (90). They suggested a stepwise CTV planning process based on their discovery that 90% of local recurrences were located within a 1-3 cm volumetric expansion from the combined celiac axis and SMA contours. Three simulated treatment plans were generated using these guidelines, and each was noticeably smaller than one generated based on recommendations per the RTOG (94).
Adding novel therapies to adjuvant chemoradiation (CRT)
Because of the limited progress made in treatment for resected pancreas cancer patients using traditional chemotherapy and CRT, novel therapeutic agents are needed.
Biologic agents that target specific molecular pathways potentially provide a novel approach in the fight against pancreas cancer (96). Investigators have developed agents against certain genes that are commonly mutated or overexpressed in pancreas cancer cells including vascular endothelial growth factor (VEGF) (97), human epidermal growth factor receptor type 2 (HER2) (98), and epidermal growth factor receptor (EGFR)/KRAS (99). While these targeted agents have shown anti-tumor activity in vitro, their clinical efficacy when added to chemotherapy has been disappointing (83,100-102). The most promising is erlotinib, a tyrosine kinase inhibitor (TKI) against ErbB1-phosphorylation (103). While it’s unclear whether the addition of erlotinib to adjuvant chemotherapy and CRT is useful (104), marginal improvements in survival have been reported by the addition of erlotinib to gemcitabine over gemcitabine alone in locally advanced and metastatic pancreas cancer patients (103). RTOG 0848 will attempt to evaluate whether erlotinib improves survival in resected pancreas patients.
Another novel adjuvant treatment approach has been to harness the body’s own immune response using vaccine therapy. Several types of vaccines have been evaluated including peptide, recombinant microorganism, and whole-cell vaccines (105). Promising results of a phase II study were published in which irradiated allogeneic granulocyte-macrophage colony stimulating factor (GM-CSF) secreting tumor vaccine was given postoperatively along with CRT (106). Hardacre et al. have described their experience using a vaccine that stimulates a hyperacute rejection-type response against two commonly expressed human pancreatic adenocarcinoma cell lines (107). In a phase II study, 70 resected pancreas patients received algenpantucel-L immunotherapy in addition to chemotherapy and CRT as per the gemcitabine arm of RTOG 9704. One-year disease free survival was 62% and OS was 86%, which paved the way for an ongoing phase III trial (NCT01072981).
Conclusions
The role of adjuvant CRT for resected pancreas cancer patients remains controversial, largely due to the conflicting results of several trials conducted decades ago that were plagued by a myriad of flaws. Studies from the modern era consistently demonstrate that adjuvant therapy, particularly including high quality RT, is beneficial especially among patients who have a particularly high risk of locoregional recurrence. In that regard, the results of RTOG 0848 are eagerly awaited. Radiation delivery techniques continue to evolve, as does our understanding of what is an appropriate adjuvant target volume, and both of these will further enhance the therapeutic ratio of RT. Lastly, novel treatments such as vaccine therapy hopefully will help us make desperately needed headway in the struggle against pancreas cancer.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Cancer Facts and Figures 2014. American Cancer Society. 2014. Available online: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2014/
- Kayahara M, Nagakawa T, Ueno K, et al. Lymphatic Flow in Carcinoma of the Distal Bile-Duct Based on a Clinicopathological Study. Cancer 1993;72:2112-7. [PubMed]
- Morganti AG, Valentini V, Macchia G, et al. Adjuvant radiotherapy in resectable pancreatic carcinoma. Eur J Surg Oncol 2002;28:523-30. [PubMed]
- Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 2000;4:567-79. [PubMed]
- Yoshida T, Matsumoto T, Sasaki A, et al. Outcome of paraaortic node-positive pancreatic head and bile duct adenocarcinoma. Am J Surg 2004;187:736-40. [PubMed]
- Hishinuma S, Ogata Y, Tomikawa M, et al. Patterns of recurrence after curative resection of pancreatic cancer, based on autopsy findings. J Gastrointest Surg 2006;10:511-8. [PubMed]
- Deki H, Sato T. An anatomic study of the peripancreatic lymphatics. Surg Radiol Anat 1988;10:121-35. [PubMed]
- Noto M, Miwa K, Kitagawa H, et al. Pancreas head carcinoma: frequency of invasion to soft tissue adherent to the superior mesenteric artery. Am J Surg Pathol 2005;29:1056-61. [PubMed]
- Whittington R, Bryer MP, Haller DG, et al. Adjuvant therapy of resected adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys 1991;21:1137-43. [PubMed]
- Kayahara M, Nagakawa T, Ueno K, et al. An evaluation of radical resection for pancreatic cancer based on the mode of recurrence as determined by autopsy and diagnostic imaging. Cancer 1993;72:2118-23. [PubMed]
- Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985;120:899-903. [PubMed]
- Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Gastrointestinal Tumor Study Group. Cancer 1987;59:2006-10. [PubMed]
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999;230:776-82; discussion 782-4. [PubMed]
- Smeenk HG, van Eijck CH, Hop WC, et al. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long-term results of EORTC trial 40891. Ann Surg 2007;246:734-40. [PubMed]
- Garofalo MC, Regine WF, Tan MT. On statistical reanalysis, the EORTC trial is a positive trial for adjuvant chemoradiation in pancreatic cancer. Ann Surg 2006;244:332-3; author reply 333. [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [PubMed]
- Choti MA. Adjuvant therapy for pancreatic cancer--the debate continues. N Engl J Med 2004;350:1249-51. [PubMed]
- Crane CH, Ben-Josef E, Small W Jr. Chemotherapy for pancreatic cancer. N Engl J Med 2004;350:2713-5; author reply 2713-5. [PubMed]
- Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol 2010;17:981-90. [PubMed]
- Kinsella TJ, Seo Y, Willis J, et al. The impact of resection margin status and postoperative CA19-9 levels on survival and patterns of recurrence after postoperative high-dose radiotherapy with 5-FU-based concurrent chemotherapy for resectable pancreatic cancer. Am J Clin Oncol 2008;31:446-53. [PubMed]
- Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 2008;299:1019-26. [PubMed]
- Regine WF, Winter KA, Abrams R, et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol 2011;18:1319-26. [PubMed]
- Van Laethem JL, Hammel P, Mornex F, et al. Adjuvant gemcitabine alone versus gemcitabine-based chemoradiotherapy after curative resection for pancreatic cancer: a randomized EORTC-40013-22012/FFCD-9203/GERCOR phase II study. J Clin Oncol 2010;28:4450-6. [PubMed]
- Miller RC, Iott MJ, Corsini MM. Review of adjuvant radiochemotherapy for resected pancreatic cancer and results from Mayo Clinic for the 5th JUCTS symposium. Int J Radiat Oncol Biol Phys 2009;75:364-8. [PubMed]
- Corsini MM, Miller RC, Haddock MG, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience (1975-2005). J Clin Oncol 2008;26:3511-6. [PubMed]
- Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol 2008;26:3503-10. [PubMed]
- Abrams RA, Winter KA, Regine WF, et al. Failure to adhere to protocol specified radiation therapy guidelines was associated with decreased survival in RTOG 9704--a phase III trial of adjuvant chemotherapy and chemoradiotherapy for patients with resected adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys 2012;82:809-16. [PubMed]
- Abrams RA, Lowy AM, O’Reilly EM, et al. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol 2009;16:1751-6. [PubMed]
- Smith RA, Bosonnet L, Ghaneh P, et al. Preoperative CA19-9 levels and lymph node ratio are independent predictors of survival in patients with resected pancreatic ductal adenocarcinoma. Dig Surg 2008;25:226-32. [PubMed]
- Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg 2003;237:74-85. [PubMed]
- Mellon EA, Springett GM, Hoffe SE, et al. Adjuvant radiotherapy and lymph node dissection in pancreatic cancer treated with surgery and chemotherapy. Cancer 2014;120:1171-7. [PubMed]
- Showalter TN, Winter KA, Berger AC, et al. The influence of total nodes examined, number of positive nodes, and lymph node ratio on survival after surgical resection and adjuvant chemoradiation for pancreatic cancer: a secondary analysis of RTOG 9704. Int J Radiat Oncol Biol Phys 2011;81:1328-35. [PubMed]
- Moghanaki D, Mick R, Furth EE, et al. Resection status, age and nodal involvement determine survival among patients receiving adjuvant chemoradiotherapy in pancreatic adenocarcinoma. JOP 2011;12:438-44. [PubMed]
- Yovino S, Poppe M, Jabbour S, et al. Intensity-modulated radiation therapy significantly improves acute gastrointestinal toxicity in pancreatic and ampullary cancers. Int J Radiat Oncol Biol Phys 2011;79:158-62. [PubMed]
- Merchant NB, Rymer J, Koehler EA, et al. Adjuvant chemoradiation therapy for pancreatic adenocarcinoma: who really benefits? J Am Coll Surg 2009;208:829-38; discussion 838-41. [PubMed]
- Richter A, Niedergethmann M, Sturm JW, et al. Long-term results of partial pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head: 25-year experience. World J Surg 2003;27:324-9. [PubMed]
- Schnelldorfer T, Ware AL, Sarr MG, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg 2008;247:456-62. [PubMed]
- You DD, Lee HG, Heo JS, et al. Prognostic factors and adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. J Gastrointest Surg 2009;13:1699-706. [PubMed]
- Hazard L, Tward JD, Szabo A, et al. Radiation therapy is associated with improved survival in patients with pancreatic adenocarcinoma: results of a study from the Surveillance, Epidemiology, and End Results (SEER) registry data. Cancer 2007;110:2191-201. [PubMed]
- Moody JS, Sawrie SM, Kozak KR, et al. Adjuvant radiotherapy for pancreatic cancer is associated with a survival benefit primarily in stage IIB patients. J Gastroenterol 2009;44:84-91. [PubMed]
- Opfermann KJ, Wahlquist AE, Garrett-Mayer E, et al. Adjuvant Radiotherapy and Lymph Node Status for Pancreatic Cancer: Results of a Study From the Surveillance, Epidemiology, and End Results (SEER) Registry Data. Am J Clin Oncol 2012. [Epub ahead of print]. [PubMed]
- House MG, Gonen M, Jarnagin WR, et al. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg 2007;11:1549-55. [PubMed]
- Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol 2006;13:1189-200. [PubMed]
- Tomlinson JS, Jain S, Bentrem DJ, et al. Accuracy of staging node-negative pancreas cancer: a potential quality measure. Archives of surgery 2007;142:767-23; discussion 773-4.
- Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol 2008;15:165-74. [PubMed]
- Berger AC, Watson JC, Ross EA, et al. The metastatic/examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg 2004;70:235-40; discussion 40. [PubMed]
- Riediger H, Keck T, Wellner U, et al. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg 2009;13:1337-44. [PubMed]
- Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery 2007;141:610-8. [PubMed]
- Benassai G, Mastrorilli M, Quarto G, et al. Factors influencing survival after resection for ductal adenocarcinoma of the head of the pancreas. J Surg Oncol 2000;73:212-8. [PubMed]
- Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg 2001;234:758-68. [PubMed]
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77. [PubMed]
- Butturini G, Stocken DD, Wente MN, et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: meta-analysis of randomized controlled trials. Arch Surg 2008;143:75-83; discussion 83. [PubMed]
- Breslin TM, Hess KR, Harbison DB, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol 2001;8:123-32. [PubMed]
- Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg 2007;246:52-60. [PubMed]
- Tummala P, Howard T, Agarwal B. Dramatic Survival Benefit Related to R0 Resection of Pancreatic Adenocarcinoma in Patients With Tumor ≤25 mm in Size and ≤1 Involved Lymph Nodes. Clin Transl Gastroenterol 2013;4:e33. [PubMed]
- Hallemeier CL, Botros M, Corsini MM, et al. Preoperative CA 19-9 level is an important prognostic factor in patients with pancreatic adenocarcinoma treated with surgical resection and adjuvant concurrent chemoradiotherapy. Am J Clin Oncol 2011;34:567-72. [PubMed]
- Ferrone CR, Finkelstein DM, Thayer SP, et al. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol 2006;24:2897-902. [PubMed]
- Lundin J, Roberts PJ, Kuusela P, et al. The prognostic value of preoperative serum levels of CA 19-9 and CEA in patients with pancreatic cancer. Br J Cancer 1994;69:515-9. [PubMed]
- Berger AC, Meszoely IM, Ross EA, et al. Undetectable preoperative levels of serum CA 19-9 correlate with improved survival for patients with resectable pancreatic adenocarcinoma. Ann Surg Oncol 2004;11:644-9. [PubMed]
- Dong M, Zhou JP, Zhang H, et al. Clinicopathological significance of Bcl-2 and Bax protein expression in human pancreatic cancer. World J Gastroenterol 2005;11:2744-7. [PubMed]
- Nio Y, Iguchi C, Yamasawa K, et al. Apoptosis and expression of Bcl-2 and Bax proteins in invasive ductal carcinoma of the pancreas. Pancreas 2001;22:230-9. [PubMed]
- Oida Y, Yamazaki H, Tobita K, et al. Increased S100A4 expression combined with decreased E-cadherin expression predicts a poor outcome of patients with pancreatic cancer. Oncol Rep 2006;16:457-63. [PubMed]
- Crane CH, Varadhachary GR, Yordy JS, et al. Phase II trial of cetuximab, gemcitabine, and oxaliplatin followed by chemoradiation with cetuximab for locally advanced (T4) pancreatic adenocarcinoma: correlation of Smad4(Dpc4) immunostaining with pattern of disease progression. J Clin Oncol 2011;29:3037-43. [PubMed]
- Herman JM, Fan KY, Wild AT, et al. Correlation of Smad4 status with outcomes in patients receiving erlotinib combined with adjuvant chemoradiation and chemotherapy after resection for pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys 2013;87:458-9. [PubMed]
- Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806-13. [PubMed]
- van der Geld YG, van Triest B, Verbakel WF, et al. Evaluation of four-dimensional computed tomography-based intensity-modulated and respiratory-gated radiotherapy techniques for pancreatic carcinoma. Int J Radiat Oncol Biol Phys 2008;72:1215-20. [PubMed]
- Kataria T, Rawat S, Sinha SN, et al. Intensity modulated radiotherapy in abdominal malignancies: our experience in reducing the dose to normal structures as compared to the gross tumor. J Cancer Res Ther 2006;2:161-5. [PubMed]
- Brown MW, Ning H, Arora B, et al. A dosimetric analysis of dose escalation using two intensity-modulated radiation therapy techniques in locally advanced pancreatic carcinoma. Int J Radiat Oncol Biol Phys 2006;65:274-83. [PubMed]
- Taylor R, Opfermann K, Jones BD, et al. Comparison of radiation treatment delivery for pancreatic cancer: Linac intensity-modulated radiotherapy versus helical tomotherapy. J Med Imaging Radiat Oncol 2012;56:332-7. [PubMed]
- Chang DS, Bartlett GK, Das IJ, et al. Beam angle selection for intensity-modulated radiotherapy (IMRT) treatment of unresectable pancreatic cancer: are noncoplanar beam angles necessary? Clin Transl Oncol 2013;15:720-4. [PubMed]
- Chang JS, Wang ML, Koom WS, et al. High-dose helical tomotherapy with concurrent full-dose chemotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2012;83:1448-54. [PubMed]
- Tunceroglu A, Park JH, Balasubramanian S, et al. Dose-painted intensity modulated radiation therapy improves local control for locally advanced pancreas cancer. ISRN Oncol 2012;2012:572342.
- Brown JM, Koong AC. High-dose single-fraction radiotherapy: exploiting a new biology? Int J Radiat Oncol Biol Phys 2008;71:324-5. [PubMed]
- Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 2009;115:665-72. [PubMed]
- Mahadevan A, Miksad R, Goldstein M, et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys 2011;81:e615-22. [PubMed]
- Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys 2013;86:516-22. [PubMed]
- Rwigema JC, Heron DE, Parikh SD, et al. Adjuvant stereotactic body radiotherapy for resected pancreatic adenocarcinoma with close or positive margins. J Gastrointest Cancer 2012;43:70-6. [PubMed]
- Morris SL, Beasley M, Leslie M. Chemotherapy for pancreatic cancer. N Engl J Med 2004;350:2713-5; author reply 2713-5. [PubMed]
- Foo ML, Gunderson LL, Nagorney DM, et al. Patterns of failure in grossly resected pancreatic ductal adenocarcinoma treated with adjuvant irradiation +/- 5 fluorouracil. Int J Radiat Oncol Biol Phys 1993;26:483-9. [PubMed]
- Yeo CJ, Abrams RA, Grochow LB, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. A prospective, single-institution experience. Ann Surg 1997;225:621-33; discussion 33-6.. [PubMed]
- Spitz FR, Abbruzzese JL, Lee JE, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol 1997;15:928-37. [PubMed]
- Hall WA, Colbert LE, Liu Y, et al. The influence of adjuvant radiotherapy dose on overall survival in patients with resected pancreatic adenocarcinoma. Cancer 2013;119:2350-7. [PubMed]
- Abrams RA, Grochow LB, Chakravarthy A, et al. Intensified adjuvant therapy for pancreatic and periampullary adenocarcinoma: survival results and observations regarding patterns of failure, radiotherapy dose and CA19-9 levels. Int J Radiat Oncol Biol Phys 1999;44:1039-46. [PubMed]
- Golden DW, Novak CJ, Minsky BD, et al. Radiation dose ≥54 Gy and CA 19-9 response are associated with improved survival for unresectable, non-metastatic pancreatic cancer treated with chemoradiation. Radiat Oncol 2012;7:156. [PubMed]
- Hanbidge AE. Cancer of the pancreas: the best image for early detection--CT, MRI, PET or US? Can J Gastroenterol 2002;16:101-5. [PubMed]
- Muler JH, McGinn CJ, Normolle D, et al. Phase I trial using a time-to-event continual reassessment strategy for dose escalation of cisplatin combined with gemcitabine and radiation therapy in pancreatic cancer. J Clin Oncol 2004;22:238-43. [PubMed]
- Ishikawa O, Ohhigashi H, Sasaki Y, et al. Practical usefulness of lymphatic and connective tissue clearance for the carcinoma of the pancreas head. Ann Surg 1988;208:215-20. [PubMed]
- Pedrazzoli S, DiCarlo V, Dionigi R, et al. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg 1998;228:508-17. [PubMed]
- Brunner TB, Merkel S, Grabenbauer GG, et al. Definition of elective lymphatic target volume in ductal carcinoma of the pancreatic head based on histopathologic analysis. Int J Radiat Oncol Biol Phys 2005;62:1021-9. [PubMed]
- Dholakia AS, Kumar R, Raman SP, et al. Mapping patterns of local recurrence after pancreaticoduodenectomy for pancreatic adenocarcinoma: a new approach to adjuvant radiation field design. Int J Radiat Oncol Biol Phys 2013;87:1007-15. [PubMed]
- Sun W, Leong CN, Zhang Z, et al. Proposing the lymphatic target volume for elective radiation therapy for pancreatic cancer: a pooled analysis of clinical evidence. Radiat Oncol 2010;5:28. [PubMed]
- Caravatta L, Sallustio G, Pacelli F, et al. Clinical target volume delineation including elective nodal irradiation in preoperative and definitive radiotherapy of pancreatic cancer. Radiat Oncol 2012;7:86. [PubMed]
- Nagakawa T, Kobayashi H, Ueno K, et al. Clinical study of lymphatic flow to the paraaortic lymph nodes in carcinoma of the head of the pancreas. Cancer 1994;73:1155-62. [PubMed]
- Goodman KA, Regine WF, Dawson LA, et al. Radiation Therapy Oncology Group consensus panel guidelines for the delineation of the clinical target volume in the postoperative treatment of pancreatic head cancer. Int J Radiat Oncol Biol Phys 2012;83:901-8. [PubMed]
- Kim K, Kim S, Chie EK, et al. Postoperative chemoradiotherapy of pancreatic cancer: what is the appropriate target volume of radiation therapy? Tumori 2005;91:493-7. [PubMed]
- Zagouri F, Sergentanis TN, Chrysikos D, et al. Molecularly targeted therapies in metastatic pancreatic cancer: a systematic review. Pancreas 2013;42:760-73. [PubMed]
- Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 2010;28:3617-22. [PubMed]
- Safran H, Iannitti D, Ramanathan R, et al. Herceptin and gemcitabine for metastatic pancreatic cancers that overexpress HER-2/neu. Cancer Invest 2004;22:706-12. [PubMed]
- Cascinu S, Berardi R, Labianca R, et al. Cetuximab plus gemcitabine and cisplatin compared with gemcitabine and cisplatin alone in patients with advanced pancreatic cancer: a randomised, multicentre, phase II trial. Lancet Oncol 2008;9:39-44. [PubMed]
- Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol 2010;28:3605-10. [PubMed]
- Pipas JM, Zaki BI, McGowan MM, et al. Neoadjuvant cetuximab, twice-weekly gemcitabine, and intensity-modulated radiotherapy (IMRT) in patients with pancreatic adenocarcinoma. Ann Oncol 2012;23:2820-7. [PubMed]
- Morgan MA, Parsels LA, Kollar LE, et al. The combination of epidermal growth factor receptor inhibitors with gemcitabine and radiation in pancreatic cancer. Clin Cancer Res 2008;14:5142-9. [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [PubMed]
- Herman JM, Fan KY, Wild AT, et al. Phase 2 study of erlotinib combined with adjuvant chemoradiation and chemotherapy in patients with resectable pancreatic cancer. Int J Radiat Oncol Biol Phys 2013;86:678-85. [PubMed]
- O’Reilly EM. Adjuvant therapy for pancreas adenocarcinoma. J Surg Oncol 2013;107:78-85. [PubMed]
- Lutz E, Yeo CJ, Lillemoe KD, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg 2011;253:328-35. [PubMed]
- Hardacre JM, Mulcahy M, Small W, et al. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 study. J Gastrointest Surg 2013;17:94-100; discussion p. 100-1.


