
Single community-based institutional series of stereotactic body radiation therapy (SBRT) for treatment of liver metastases
Introduction
Aggressive local treatment with surgical resection of oligometastatic liver metastases results in long-term survival in many patients (1). However, not all patients are medically suitable for metastasectomy or partial hepatectomy. Alternative non-surgical ablative approaches for treatment of isolated liver metastases have been developed, including radiofrequency ablation, cryotherapy, and yttrium 90 microsphere therapy. One such technology, stereotactic body radiation therapy (SBRT), has become an increasingly important option in the treatment of liver metastases as it focuses a high dose of radiation to a small planning target volume (PTV) while sparing the majority of liver tissue (2,3).
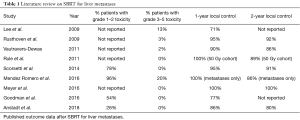
SBRT has been shown to be a safe and effective non-invasive option for treatment of liver metastases through both retrospective and phase I/II prospective studies that show 2-year local control (LC) ranging from 67% to 100% (Table 1) (4-13). A phase I/II prospective trial conducted by Rusthoven et al. showed LC at 1 and 2 years to be 95% and 92%, respectively. Lesions with a maximum diameter of 3 cm or less had a 2-year LC of 100%, while lesions with a maximum diameter greater than 3 cm had 2-year LC of 77%. Median progression free survival (PFS) was 6.1 months and the 2-year overall survival (OS) rate was 30%. In this study, toxicity was minimal—1 patient developed a grade 3 soft tissue toxicity, and no grade 4 or 5 toxicities were reported. When analysis was limited to only metastatic lesions, Toesca et al. found 11.7% of patients developed grade 3 toxicities including ALT/AST elevation with one death related to hepatobiliary toxicity.
Full table
When considering the implementation of SBRT for the treatment of liver metastases, it is notable that most of these current published reports are from high-volume referral centers that perform large numbers of these procedures (14,15). Some studies have suggested that patients who undergo technically challenging surgery for advanced cancers at high-volume centers have better overall survival when compared to low-volume centers (16-22). Further, some have suggested that, analogous to surgery, advanced radiotherapy techniques performed at low-volume centers result in inferior outcomes and in head and neck cancer was even shown to negatively affect overall survival of patients (23). Others have recently asserted that certain cancers should be preferentially treated at academic centers because treatment at community hospitals was found to be associated with an increased risk of death (24-27).
Implications of this assertion are wide-ranging and therefore, additional studies on the implementation of advanced radiotherapy techniques in these various settings are warranted. To our knowledge there are no studies that demonstrate significantly different liver SBRT outcomes based on the experience or volume of the treating cancer center. In this study, we assess SBRT for the treatment of liver metastases at a low-volume, community-based hospital by studying the efficacy and toxicity in 42 consecutively treated patients over a 10-year period through the use of retrospective, database analysis.
Methods
Study population
The patient cohort used in this study consisted of subjects entered into an institutional review board approved registry at a community-based hospital. Written informed consent was obtained from patients undergoing stereotactic body radiotherapy for liver metastases from various primary tumor sites between 2006 and 2016. Patients were then entered into the registry, which was periodically updated to reflect a current status of treatment outcomes. Patients with primary liver neoplasms were excluded from analysis.
Treatments
All patients underwent fiducial marker placement under CT-guidance 1–2 weeks prior to planning scans. Gross tumor volume (GTV) was delineated using contrast enhanced CT scans, as well as fusion with positron emission tomography (PET) and/or magnetic resonance imaging (MRI) scans. GTV was expanded by 5 mm to create the PTV. Treatment was delivered by image guided stereotactic robotic radiosurgery with respiratory motion tracking. Lesions were treated with 3 consecutive fractions to a median total dose of 54 Gy.
Data collection
Data from each patient was collected and entered into the patient registry database. These data consisted of age, sex, primary tumor location and control status, time since diagnosis of primary tumor, prior local treatment, number of liver metastases, presence of extrahepatic metastases, Karnofsky performance score (KPS) prior to treatment, maximum diameter of lesion treated, GTV volume, total dose, isodose line to which dose was prescribed and fractions used, date of treatment and date of latest follow-up, overall cancer status, maximum local control achieved (stability, partial regression or complete regression), local control status and last date with local control, as well as toxicity reported. Radiation toxicities were graded according to the Radiation Therapy and Oncology Group (RTOG) common toxicity criteria.
Statistical analysis
Forty-two consecutively treated patients with 81 individual liver lesions were analyzed. Outcomes measured were 1- and 2-year overall survival (OS), progression-free survival (PFS) and local failure-free survival. All outcomes were defined as the time from the date of delivery of the first fraction of treatment to death (OS), progression of disease at any site (PFS), or local progression of the treated liver lesion (local failure-free survival). Other outcomes measured were maximum local control achieved, presence of and time to new liver metastases and toxicity grade and frequency. Overall survival, PFS and local failure-free survival were estimated using Kaplan-Meier method. To investigate the relationship between tumor volume and local control, we dichotomized our data set at the mean. Log-rank statistic was used to compare local control based on gross tumor volume. All statistical analyses were carried out using SPSS version 20 (IBM Corp., Armonk, NY, USA). Statistical tests were considered significant at P values <0.05.
Results
Patient and disease characteristics
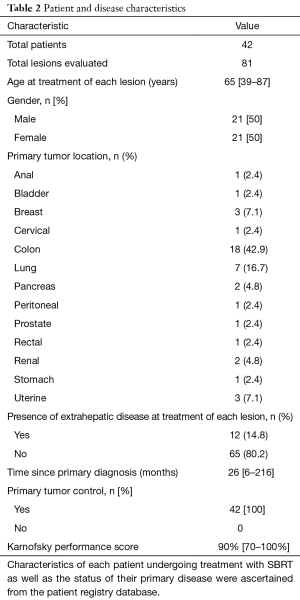
Between 2006 and 2016, 42 consecutively treated patients with a total of 81 metastatic liver lesions were treated with SBRT at this non-academic, community hospital (Table 2). The median age at treatment was 65 years old and ranged from 39 to 87 years old. The patient population consisted of 50% female and 50% male patients. The primary tumor site was colon for 18 patients (42.9%), lung for 7 patients (16.7%), breast for 3 patients (7.1%) and uterine for 3 patients (7.1%). Other primary sites consisted of anal, bladder, cervical, peritoneal, prostate, rectal, renal, pancreas and stomach cancers. At the time of SBRT, the median elapsed time from primary diagnosis was 26 months and ranged from 6 to 216 months. Synchronous extrahepatic disease was present with 14.8% of the treated lesions at the time of SBRT. The KPS score was at least 70 (median of 90) in each patient treated. The primary tumor was controlled in all patients at the time of SBRT.
Full table
Lesion and treatment characteristics
Of the 81 total lesions treated with SBRT, 33.4% had prior local treatment for liver metastases at a different liver site consisting of SBRT in 19.8%, resection in 2.5%, cryoablation in 1.2%, and multiple modalities in 9.9% (Table 3). The majority (66.7%) had no prior local treatment. The median number of lesions that were treated at one time was 1 and ranged from 1–4. The median number of lesions treated per patient was also 1 but ranged from 1–6. Some of these patients developed new liver metastases years after treatment and had additional treatment with SBRT. Lesions had a median maximum diameter of 2.5 cm (range, 0.5–9.5 cm), and a mean volume of 53 cc (range, 0.5–363.0 cc). Three fractions were used for all treatments and a dose of 14–20 Gy (median 18 Gy) per fraction was prescribed to a median isodose line of 78% (range, 56–86%).

Full table
Treatment outcomes
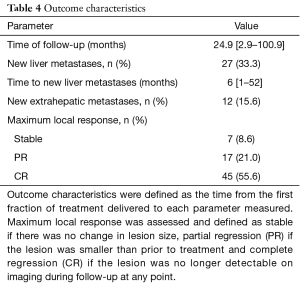
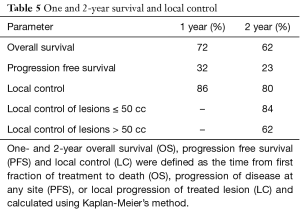
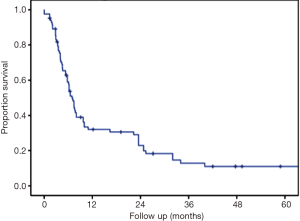
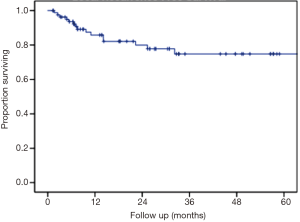
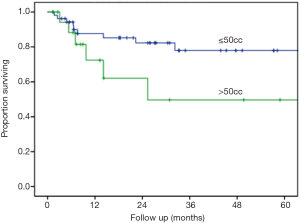
The median follow up was 24.9 months and the longest follow-up time was 100.9 months (Table 4). During follow-up, 33.3% developed new liver metastases with a median time to new liver metastases of 6 months after treatment. In addition, 15.6% of patients developed new extrahepatic metastases during follow-up. The maximum local response rate was complete regression in 55.6% of treated lesions, partial regression in 21% of treated lesions, and stability in 8.6% of treated lesions (Table 4). Local failures occurred in 12 lesions. However, most patients who experienced local failure had poor initial follow-up and eventual compliance was many months or even years later, introducing the potential for undetected treatment success in some individuals. Only 2 of these 12 lesions progressed within the first 3 months after treatment, representing true non-responders. Kaplan-Meier estimated 1- and 2-year outcomes are compiled in Table 5. Estimated 1- and 2-year overall survival was 72% and 62%, respectively (Figure 1). Estimated 1- and 2-year progression free survival was 32% and 23%, respectively (Figure 2). Estimated 1- and 2-year local control was 86% and 80%, respectively (Figure 3). Two-year local control was significantly lower for lesions >50 cc compared to lesions ≤50 cc (62% vs. 84%, P=0.04; Figure 4). Although the two lesions that progressed within three months of treatment were both from primary colon cancer.
Full table
Full table

Safety
The majority (71.9%) of treatments were not associated with any toxicity. Toxicity did occur in 26.4% of treatment courses with grade 1 toxicity (n=12) including fatigue, diarrhea, nausea, skin irritation and pneumonitis reported as well as grade 2 toxicity (n=3) with chest wall pain and vomiting with mildly elevated transaminase levels (Table 6). Two of the occurrences of grade 2 toxicity were in the same patient treated again years later for new liver metastases. No other side effects were reported.

Full table
Conclusions
The concept of regionalized care, where patients travel from local hospitals to regional hospitals to receive care, has gone in and out of favor over the last several decades (22). More recently, studies have found that low-volume or non-academic institutions may produce inferior outcomes when it comes to technically challenging radiotherapy treatments (24-27). Some have even suggested that the most complex cases should routinely be referred for treatment exclusively at high volume centers. These data were somewhat concerning, since we perform many technically complex treatments, including SBRT, in our non-academic low-volume community based hospital.
In the present study, we applied these questions to our 10-year patient registry database to evaluate the efficacy and safety of SBRT for liver metastases at a low-volume, community-based hospital. To accurately determine the treatment outcomes of SBRT for liver metastases, we excluded patients with primary liver cancer from this study. Additionally, we focused our treatment plans to 3 fractions for all patients and kept the total dose within a tight range. The results found are not only comparable to available published data at academic institutions regarding the efficacy of this technology but also show a low toxicity profile. Current published studies from high-volume institutions on SBRT for liver metastases show LC at 2 years ranges from 86% to 100% (Table 1) (4-10,12-14). In this analysis, LC at 2 years was found to be 80%. In the phase I/II prospective trial published by Rustheven et al., the LC rate at 2 years was 92%, however, this study did not include 7 patients who died prior to analysis of LC. In this analysis, smaller lesions treated resulted in better local control (lesions ≤50 cc showed 2 year LC of 84%) as is also seen in the available published literature (Rustheven et al. showed lesions ≤3 cm with 2-year LC of 100%). We found that toxicities associated with SBRT for liver metastases did not exceed grade 2, while previously published studies showed examples of grade 3–5 toxicities including soft tissue toxicities, gastritis, nausea, radiation pneumonitis and radiation-induced liver disease (6,14,28). Studies that have found disparities between high volume and low volume centers acknowledge that demographic differences may at least in part account for the difference in outcomes. Subtle differences in demographics that relate to social support and financial stability are difficult to measure and are beyond the scope of this study. However, insofar as these factors do matter, at our institution we are able to achieve excellent results despite demographic differences unique to our community that could lead to health disparities. Overall, this analysis shows that SBRT for the treatment of oligometastatic liver metastases at our single-institution is an effective and safe option with outcomes that are comparable to high-volume institutions.
Some complex cases should be referred to high volume centers. For instance, referrals are appropriate when standard of care technology or subspecialty training are not available locally. Indeed, it is our practice to refer for second opinion when appropriate. On numerous occasions our patients have benefitted greatly from specialized care at regional centers not available in our community. However, there are costs associated with referral to high volume centers. For many patients, traveling long distances to receive radiation treatment may simply not be logistically feasible. Additionally, financial costs associated with transportation, food and housing may either be prohibitive or at least a significant source of stress. Further, out of pocket costs of medical care can be limiting for patients who seek treatment outside their network of providers. Finally, for patients with metastatic cancer, quality time spent with family is typically a high priority and traveling for treatment may introduce excess psychological morbidity related to feelings of isolation and missed opportunities for companionship. Therefore, if outcomes are comparable, then it is preferable for patients to undergo complex treatments closer to home.
Our results should not be applied broadly across community-based practices. Instead, we recommend community practices evaluate their own outcomes for complex radiation treatments in the context of available data from high volume centers. For centers with inferior results or with limited experience, disparities in outcomes may be reduced by utilizing online contouring resources like econtour.org, tele-oncology, remote planning and immersion courses sponsored by medical specialty societies. Radiation oncology is a unique specialty in which treatments are planned and delivered entirely through computer-based programs. Unlike surgery, where proficiency can only be achieved through participating in the operating room, radiation therapy lends itself to online training tools. There is fertile ground for the expansion of such programs either in the private sector, or through our professional societies.
We believe it is imperative that we maintain high quality programs at low-volume centers to provide state-of-the-art technology directly to the communities that we serve. Over the last decade, we have developed a stereotactic radiation program that adheres to American Association of Physicists in Medicine standards and is American College of Radiology certified. All physicians in our group are certified by the American Board of Radiology. Dissemination of the concept that, despite adherence to these standards and certifications, our patients are best served by referral high volume centers for complex treatments will erode the relevance and necessity of such standards and certifications. In this scenario, patient safety will decline. Instead, we advocate for more active engagement between the academic community and accrediting bodies with community-based practices to establish standards of care for complex treatments. The alternative—routine referral to high volume centers—will inevitably widen the gap where disparities do exist.
The strengths of this study are the consistency of the treatment parameters each patient received, the specificity of the liver lesion being metastatic and not primary liver cancer, and the long follow-up time achieved for many of the patients in this study. The limitation of this study is the modest patient population at a single institution. However, for our goal of determining the efficacy and safety of SBRT for liver metastasis in a small-volume, community-based hospital, these data are compelling. These findings demonstrate the successful implementation of a liver metastasis SBRT program in a low-volume setting. Therefore, at this institution, referral to a high volume academic center for liver metastasis SBRT is likely not worth the monetary cost and inconvenience. This work suggests that while referral to high-volume centers may be justified for certain treatments, that each situation warrants investigation to determine if the benefits associated will outweigh the costs incurred by the patients living in the communities we serve.
Acknowledgements
We would like to acknowledge Laura Patrick and the Cyberknife Center at Saint Francis Hospital.
Footnote
Conflicts of Interest: The results of this study were in part presented at the 2017 RSNA Annual meeting. The authors have no conflicts of interest to declare.
Ethical Statement: The IRB approval numbers for the data collection and analysis of this database are: #07-10-003 and #SFH-17-13. All subjects who were able, signed the informed consent forms before enrollment. Deceased patients whose information was included were retrieved from medical records and all personal data have been secured.
References
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21. [Crossref] [PubMed]
- Kress MS, Collins BT, Collins SP, et al. Stereotactic body radiation therapy for liver metastases from colorectal cancer: analysis of safety, feasibility, and early outcomes. Front Oncol 2012;2:8. [Crossref] [PubMed]
- Scorsetti M, Clerici E, Comito T. Stereotactic body radiation therapy for liver metastases. J Gastrointest Oncol 2014;5:190-7. [PubMed]
- Goodman BD, Mannina EM, Althouse SK, et al. Long-term safety and efficacy of stereotactic body radiation therapy for hepatic oligometastases. Pract Radiat Oncol 2016;6:86-95. [Crossref] [PubMed]
- Herfarth KK, Debus J, Lohr F, et al. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol 2001;19:164-70. [Crossref] [PubMed]
- Lee MT, Kim JJ, Dinniwell R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol 2009;27:1585-91. [Crossref] [PubMed]
- Méndez Romero A, de Man RA. Stereotactic body radiation therapy for primary and metastatic liver tumors: From technological evolution to improved patient care. Best Pract Res Clin Gastroenterol 2016;30:603-16. [Crossref] [PubMed]
- Meyer JJ, Foster RD, Lev-Cohain N, et al. A Phase I Dose-Escalation Trial of Single-Fraction Stereotactic Radiation Therapy for Liver Metastases. Ann Surg Oncol 2016;23:218-24. [Crossref] [PubMed]
- Milano MT, Katz AW, Muhs AG, et al. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer 2008;112:650-8. [Crossref] [PubMed]
- Rule W, Timmerman R, Tong L, et al. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol 2011;18:1081-7. [Crossref] [PubMed]
- Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol 2009;27:1579-84. [Crossref] [PubMed]
- Scorsetti M, Comito T, Tozzi A, et al. Final results of a phase II trial for stereotactic body radiation therapy for patients with inoperable liver metastases from colorectal cancer. J Cancer Res Clin Oncol 2015;141:543-53. [Crossref] [PubMed]
- Vautravers-Dewas C, Dewas S, Bonodeau F, et al. Image-guided robotic stereotactic body radiation therapy for liver metastases: is there a dose response relationship? Int J Radiat Oncol Biol Phys 2011;81:e39-47. [Crossref] [PubMed]
- Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009;27:1572-8. [Crossref] [PubMed]
- Toesca DA, Osmundson EC, Eyben RV, et al. Central liver toxicity after SBRT: An expanded analysis and predictive nomogram. Radiother Oncol 2017;122:130-6. [Crossref] [PubMed]
- Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998;280:1747-51. [Crossref] [PubMed]
- Bilimoria KY, Talamonti MS, Sener SF, et al. Effect of hospital volume on margin status after pancreaticoduodenectomy for cancer. J Am Coll Surg 2008;207:510-9. [Crossref] [PubMed]
- Birkmeyer JD, Finlayson SR, Tosteson AN, et al. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery 1999;125:250-6. [Crossref] [PubMed]
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128-37. [Crossref] [PubMed]
- Birkmeyer JD, Warshaw AL, Finlayson SR, et al. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery 1999;126:178-83. [Crossref] [PubMed]
- Lüchtenborg M, Riaz SP, Coupland VH, et al. High procedure volume is strongly associated with improved survival after lung cancer surgery. J Clin Oncol 2013;31:3141-6. [Crossref] [PubMed]
- Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med 1979;301:1364-9. [Crossref] [PubMed]
- Wuthrick EJ, Zhang Q, Machtay M, et al. Institutional clinical trial accrual volume and survival of patients with head and neck cancer. J Clin Oncol 2015;33:156-64. [Crossref] [PubMed]
- Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: importance in quality of cancer care. J Clin Oncol 2000;18:2327-40. [Crossref] [PubMed]
- Lin JF, Berger JL, Krivak TC, et al. Impact of facility volume on therapy and survival for locally advanced cervical cancer. Gynecol Oncol 2014;132:416-22. [Crossref] [PubMed]
- Zumsteg ZS, Luu M, Yoshida EJ, et al. Combined high-intensity local treatment and systemic therapy in metastatic head and neck squamous cell carcinoma: An analysis of the National Cancer Data Base. Cancer 2017;123:4583-93. [Crossref] [PubMed]
- Yoshida EJ, Luu M, David JM, et al. Facility Volume and Survival in Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys 2018;100:408-17. [Crossref] [PubMed]
- Hijazi H, Campeau MP, Roberge D, et al. Stereotactic Body Radiotherapy for Inoperable Liver Tumors: Results of a Single Institutional Experience. Cureus 2016;8:e935. [PubMed]


